Cathepsin B cleavable linker is a widely used class of enzyme-sensitive linkers that achieve efficient and precise drug release by leveraging the high expression of Cathepsin B in tumor cell lysosomes. BOC Sciences, relying on an advanced bioconjugation technology platform and extensive experience in antibody-drug conjugate (ADC) development, provides custom development services for Cathepsin B cleavable linkers to clients worldwide. Our services cover the full process, including linker sequence design, chemical synthesis, conjugation strategy optimization, and stability validation, helping customers accelerate the R&D of ADCs and related bioconjugates.
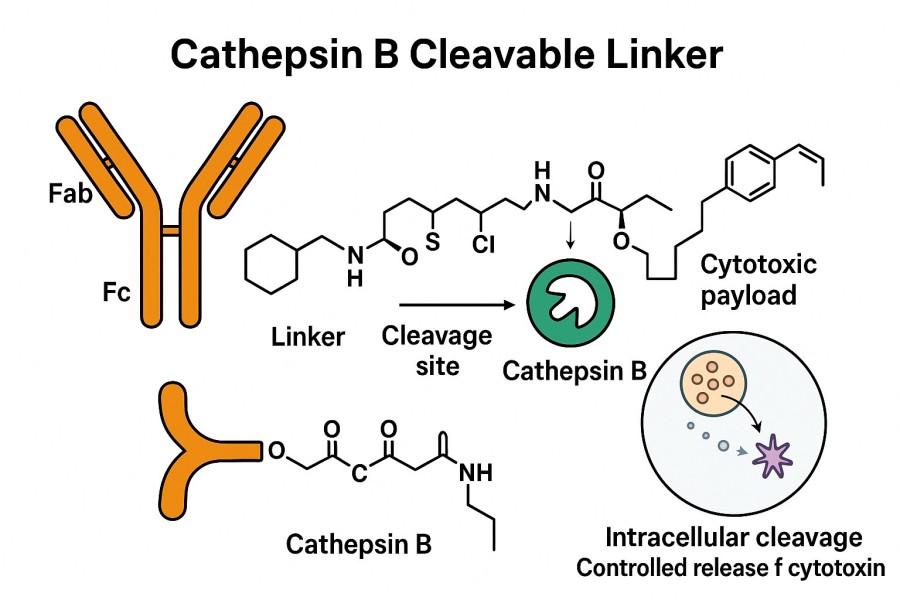
Cathepsin B is a cysteine protease primarily located in the lysosomes of cells. Its expression is significantly elevated in various solid tumors and hematologic malignancies, and it can exert hydrolytic activity both inside and outside tumor cells. This characteristic makes it an important target enzyme for ADC linker design. In Cathepsin B-cleavable ADCs, the cleavable linker typically contains a specific Cathepsin B cleavage site (such as the dipeptide sequences Val-Cit or Val-Ala) and a self-immolative spacer group (PABC, p-aminobenzyl carbamate). The core principle is to utilize the highly expressed Cathepsin B protease within tumor cell lysosomes to cleave the specific peptide sequence, after which the PABC group undergoes self-immolation, rapidly releasing the active drug molecule. This enhances tumor killing efficacy while reducing toxicity to normal tissues.
BOC Sciences, based on a mature bioconjugation platform and years of ADC development experience, offers one-stop custom services for Cathepsin B cleavable linkers from molecular design to scale-up production. Our services cover not only conventional linker design and synthesis but also process optimization, functional validation, and subsequent batch manufacturing to ensure your ADC projects advance efficiently and deliver stably.
We provide fully customized linker sequence design according to the structural features of your drug, the type of targeting antibody, and the intended indication, with computational chemistry prediction performed in advance to improve R&D efficiency.
BOC Sciences possesses a comprehensive peptide synthesis platform capable of efficiently producing high-purity, highly consistent Cathepsin B cleavable linkers:
The ultimate goal of the linker is to achieve efficient and controlled attachment of small molecule drugs to antibodies. BOC Sciences offers full-process conjugation development:
To ensure linker performance stability both in vitro and in vivo, we provide multi-dimensional functional validation services:
BOC Sciences leverages its profound ADC development experience and advanced peptide chemistry platform to offer customers tailored Cathepsin B cleavable linker solutions specific to their antibodies and payloads. We understand that the structural features of different antibodies (such as modification site distribution and hydrophilic/hydrophobic balance) and the chemical properties of payloads (such as solubility, stability, nucleophilicity/electrophilicity) directly impact linker design and performance. Therefore, we achieve high-precision customization through the following strategies:
BOC Sciences has accumulated years of practical experience in ADC and linker R&D, successfully supporting numerous international projects. We are well-versed in key design aspects of ADC linkers and can provide precise optimization tailored to different drugs and antibodies.
Our company is equipped with peptide synthesis, antibody modification, conjugation process development, and advanced analytical instruments such as HPLC, MS, and NMR, offering comprehensive technical support for structural validation, purity analysis, and functional evaluation of Cathepsin B cleavable linkers.
We tailor linker sequences, functional groups, and hydrophilicity adjustments based on clients' drug types, targeting strategies, and pharmacokinetic needs to ensure optimal Cathepsin B cleavable linker performance in specific applications.
Manufacturing complies with cGMP and ISO international quality standards, ensuring each batch of Cathepsin B cleavable linkers maintains high consistency in purity, stability, and functionality, accompanied by complete analytical reports and regulatory documentation.
We provide full-process support from laboratory research, pilot trials, and scale-up to commercial production, guaranteeing process stability and cost control at every stage of Cathepsin B cleavable linker manufacturing.
BOC Sciences' project teams offer cross-timezone technical communication and support for clients worldwide, responding promptly to R&D needs and shortening the cycle from design to delivery of Cathepsin B cleavable linkers.

Engage in in-depth discussions with clients to clarify project goals, drug types, and antibody characteristics, defining functional requirements for the Cathepsin B cleavable linker, and conducting feasibility analysis and preliminary technical assessment.
Design appropriate peptide sequences, self-immolative groups, and functional modifications based on Cathepsin B substrate characteristics to ensure an optimal balance between stability and enzymatic cleavage efficiency.
Utilize solid-phase peptide synthesis and precise purification methods to prepare linkers, confirming Cathepsin B cleavable linker purity and structure via HPLC, MS, and other analytical techniques.
Select suitable antibody modification sites and conjugation chemistries, control DAR values, and optimize reaction conditions to ensure efficient conjugation of linker, drug, and antibody with preserved function.
Conduct serum stability, enzymatic cleavage efficiency, and drug release rate testing, alongside cellular assays to validate targeted release performance of the Cathepsin B cleavable linker.

Complete process scale-up and batch production under cGMP conditions, providing comprehensive quality reports and technical support to ensure timely and high-quality delivery of Cathepsin B cleavable linkers.
Cathepsin B is a lysosomal cysteine protease highly upregulated in malignant cells, making it attractive for prodrug activation. Notably, its plasma concentration is lower than in lysosomes. Its activity peaks at acidic pH, while the slightly basic pH of circulation keeps the enzyme inactive, avoiding premature drug release. Cathepsin B recognizes and cleaves specific dipeptide sequences. Preliminary screening showed efficient doxorubicin release from certain dipeptides after Cathepsin B incubation. Among more than 10 dipeptides screened, the Val-Cit linker demonstrated the greatest potential due to protease recognition and stability in human and mouse serum. Consequently, Val-Cit linkers are widely used in enzymatically cleavable ADC linkers. Approximately 20% of ADCs in clinical trials incorporate a Val-Cit linker to control drug release.
Cathepsin B mainly cleaves peptide bonds at specific amino acid sequences, commonly at dipeptides such as Val-Cit and Val-Ala. By recognizing and cleaving these Cathepsin B cleavage sites, ADCs release the drug within lysosomes. This cleavage mechanism ensures precise drug release inside tumor cells, enhancing therapeutic efficacy while reducing side effects.
These linkers exploit the high intracellular expression of Cathepsin B in tumor cells for specific drug release, improving ADC targeting and therapeutic outcomes while reducing toxicity to normal tissues. They exhibit high blood stability and efficient cleavage.
Cathepsin B cleavable linkers are a specific subset of peptide linkers designed with peptide sequences recognized and cleaved specifically by Cathepsin B (e.g., Val-Cit), releasing drugs inside tumor cell lysosomes. Peptide linkers broadly refer to any amino acid chain-based linkers, which may not necessarily be sensitive to a specific enzyme. In short, all Cathepsin B cleavable linkers are peptide linkers, but not all peptide linkers are Cathepsin B cleavable.
Peptide-based linkers are designed to maintain ADC integrity in systemic circulation and allow rapid release of cytotoxic drugs upon cleavage by specific intracellular proteases like Cathepsin B. Due to unsuitable pH and serum protease inhibitors, peptide linkers offer good systemic stability and fast enzymatic cleavage in target cells. Examples include Val-Cit and Phe-Lys dipeptide linkers, both clinically used and balancing plasma stability with intracellular protease cleavage.
The Val-Cit dipeptide is one of the most commonly used cleavable linkers in ADCs, with multiple clinical-stage candidates due to its plasma stability, release behavior, and chemical tractability. Two approved ADC drugs (ADCETRIS and POLIVY) utilize the mc-VC-PABC linker construct containing a maleimidocaproyl spacer, the Val-Cit dipeptide as Cathepsin substrate, and a PABC self-immolative spacer. The Val-Ala dipeptide is also widely used; for example, Loncastuximab Tesirine includes a pegylated spacer to balance payload lipophilicity. The Val-Ala linker enables DAR up to 7.4 with limited aggregation (<10%) and suits lipophilic payloads like PBD dimers.
We precisely design Cathepsin B cleavage site sequences and self-immolative groups by integrating client antibody structures and payload physicochemical properties, optimizing linker length and functional groups to ensure in vivo stability and high cleavage efficiency for customized, efficient conjugation.
BOC Sciences employs advanced analytical techniques including HPLC, mass spectrometry (MS), and nuclear magnetic resonance (NMR) to rigorously assess linker purity, structural integrity, and conjugation efficiency, ensuring each batch meets cGMP and international quality standards with comprehensive analytical reports.
Depending on project complexity, initial design and small-scale synthesis usually take 4–8 weeks. Subsequent conjugation optimization and functional validation require about 4–6 weeks. Scale-up production and batch delivery times are flexible to meet client schedules, ensuring timely R&D progress and quality.
Background
A European biopharmaceutical company developing a novel ADC for solid tumors faced the core challenge of designing a linker with both high plasma stability and efficient intracellular drug release. Initially, they used commercially available generic linkers, but in vitro plasma stability assays showed premature degradation causing increased off-target cytotoxicity and unsatisfactory conjugation efficiency with their specific antibody. Therefore, the company partnered with BOC Sciences to custom develop a Cathepsin B cleavable linker adapted to their antibody and payload.
How BOC Sciences Helped
BOC Sciences first performed in-depth analysis of the antibody structure and drug molecule to identify suitable conjugation sites and chemical reaction pathways. Based on Cathepsin B substrate specificity, a linker containing a Val-Cit dipeptide and a self-immolative spacer was designed to ensure rapid drug release. The linker's hydrophilicity was adjusted to improve overall ADC solubility and in vivo stability.
Implementation
Results
Multiple scientific publications have utilized linkers provided by BOC Sciences, demonstrating their critical role in ADC and bioconjugate design and development. Clients have successfully completed drug release mechanism validation, cleavage efficiency evaluation, and cell targeting studies using our high-quality linkers, with results published in internationally recognized journals.

"BOC Sciences demonstrated exceptional professionalism in the custom development of Cathepsin B cleavable linkers. The designed linkers significantly improved the drug release efficiency of our ADCs. Communication was smooth, technical support was timely, and the overall collaboration experience was excellent."
— Dr. Emily Chen, R&D Director (USA)
"They custom-designed a linker that precisely met our requirements for stability and enzymatic cleavage specificity. Their conjugation process optimization was outstanding, substantially shortening our project development timeline."
— Mr. James Müller, Head of Antibody Drug Development (Germany)
"The team conducted in-depth analysis of our antibody structure and payload, providing personalized design solutions. The product quality was consistent and delivery punctual, offering solid support for our preclinical studies."
— Dr. Aisha Rahman, Project Manager (Singapore)
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










