β-Glucuronidases are a class of glycosidase enzymes that catalyze β-glucuronide residues hydrolysis and are widely used in antibody-drug conjugates (ADCs) design. BOC Sciences, leveraging its strong chemical synthesis expertise, extensive conjugation experience, and advanced R&D facilities, provides high-quality custom synthesis services of β-glucuronide linkers to global clients, assisting research institutions and biopharmaceutical companies in accelerating the development of innovative drugs.
β-Glucuronidases are a class of hydrolytic lysosomal enzymes that degrade β-glucuronic acid residues into polysaccharides. β-glucuronidases are found exclusively in the lysosomal compartment of the cell and work under hydrophilic environments to release payloads from conjugates. Similar to cathepsin B, β-glucuronidases are secreted in necrotic areas of certain tumors. Using highly potent microtube inhibitors (MMAE and MMAF) and a DNA-damaging agent (doxorubicin), β-glucuronidase-susceptible ADCs were designed by covalent conjugation of cytotoxic agents to the antibody via a β-glucuronide linker attached to a self-immolative PABC spacer molecule. The introduction of a self-immolative spacer enhances linker stability and allows the safe release of potent cytotoxins. Therefore, drug release proceeds in two steps: (i) enzymatic hydrolysis of the glycosidic bound; and (ii) spontaneous decomposition of the linker, leading to the release of active compounds.
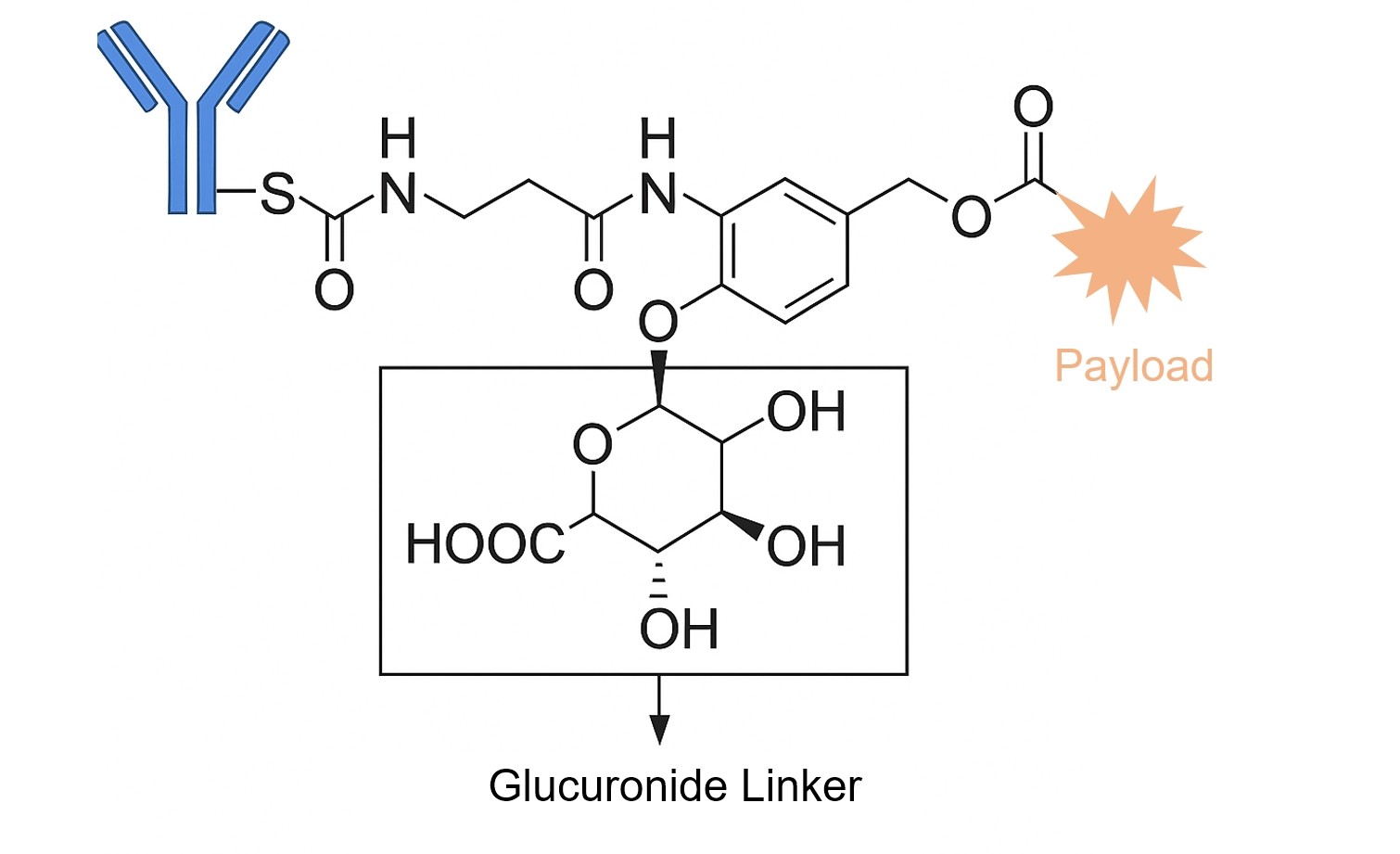 Fig. 1. ADC containing glucuronide linker (BOC Sciences Authorized).
Fig. 1. ADC containing glucuronide linker (BOC Sciences Authorized).
The β-glucuronidases abundance in lysosomes and tumor interstitium associated with the hydrophilicity of its substrates are the reasons of interest for designing safe and efficient cleavable ADC linkers. Such linkers demonstrated a low level of aggregation, high plasma stability, a cleavage process validated in vitro upon incubation with corresponding enzymes, and robust in vivo efficacy. The β-glucuronide linker has also been applied to other amine-containing payloads such as CBI minor groove binder, camptothecin analogs, and hydroxyl-containing molecules SN38, duocarmycin, and psymberin via an additional dimethyl ethylene diamine (DMED) self-immolative spacer. An additional cyclization of DMED occurs spontaneously to form 1,3-dimethylimidazolin-2-one and releases the hydroxyl-containing drug eventually. Due to the hydrophilicity of linkers, this technology allows easier preparation of ADCs with DAR 8 compared to cathepsin-sensitive linkers.
BOC Sciences' β‑glucuronide linker synthesis services center on customers' core needs in ADC development, offering a one-stop solution from design and custom synthesis to quality verification and process scale-up. We focus on addressing challenges faced by traditional linkers such as plasma stability, drug-to-antibody ratio (DAR) control, and aggregation, helping clients develop safer and more efficient next-generation ADC products.
BOC Sciences possesses extensive expertise in polysaccharide chemistry and complex small molecule synthesis, enabling us to custom synthesize diverse β‑glucuronidase-sensitive linkers according to client-specified design requirements. We can synthesize linkers featuring various self-immolative spacers (such as the classical PABC or novel DMED) and diversified terminal functional groups to accommodate different payloads (MMAE, MMAF, Doxorubicin, Camptothecin, etc.). Additionally, our team can explore novel glycosidase-sensitive structures based on the latest literature or client innovations, advancing cutting-edge ADC research and development.
The success of linker design is directly linked to ADC stability and therapeutic efficacy. BOC Sciences provides professional structural optimization services to help clients adjust linker polarity, length, and chemical stability to ensure efficient conjugation with the target payload while maintaining plasma stability to prevent premature drug release. For small molecule drugs containing multiple functional groups such as phenolic hydroxyl, amine, and carboxyl groups, we precisely select the optimal conjugation strategy to avoid side reactions and enhance overall conjugation efficiency.
From milligram-scale laboratory synthesis to gram- and kilogram-scale production needed for preclinical and clinical stages, BOC Sciences offers comprehensive process development and scale-up support. Our process development team specializes in shortening synthesis routes, optimizing reaction conditions, and reducing raw material costs to provide clients with efficient synthetic pathways that meet industrialization requirements. Furthermore, BOC Sciences can conduct process validation in a GMP-like environment, laying a solid foundation for subsequent GMP production and ensuring products meet stringent regulatory standards.
High-quality linkers must undergo multi-dimensional testing and validation. BOC Sciences is equipped with advanced analytical platforms, including Nuclear Magnetic Resonance (NMR), Mass Spectrometry (MS), High-Performance Liquid Chromatography (HPLC), and Liquid Chromatography-Mass Spectrometry (LC-MS). We perform structural confirmation, purity analysis, impurity profiling, and stability testing, providing detailed analytical reports to ensure every batch of linkers meets the highest quality standards. Through systematic quality control, clients can confidently use our linkers for subsequent antibody conjugation and in vitro/in vivo efficacy evaluations.
Due to their enzyme-specific cleavage and drug release properties in the tumor microenvironment, β-glucuronide linkers have become a key component in ADC design. BOC Sciences offers customized design services integrating small molecule drug activity optimization, antibody conjugation stability enhancement, and target specificity strategies, committed to advancing efficient and safe precision drug delivery solutions.
Apart from using auristatin and doxorubicin, BOC Sciences provides β-glucuronide linker customized-design services targeting special cytotoxic agents such as anthracyclines, camptothecin derivatives, taxanes, hedgehog inhibitors, nitrogen mustards, and histone deacetylase inhibitors. Most of our drug conjugation strategies contain a self-immolative PABC spacer molecule between the β-glucuronide linker and drug moiety, thereby offering a straightforward payload release upon internalization.
The upregulated hydrophilicity of β-glucuronic acid glycoside linkers increases the stability of ADCs. BOC Sciences has experts in small molecule drug development and developed a variety of β-glucuronide linkers for specific antibodies based on customers' new drug development. Furthermore, our one-stop service platform supports a wide range of antibody modification and conjugation services to meet your research needs.

The introduction of a glycosidase cleavable linker featuring a hydrophilic sugar moiety also helps overcome aggregation issues. The resulting ADC avoided common problems arising from high DARs, such as aggregation and faster clearance. Within this context, β-glucuronide linkers have also been used for the controlled release of cytotoxic drugs in various targets. In BOC Sciences, our scientists can design the most suitable linker for tumor targets based on the drug release mechanism and physical properties of the linker itself, thereby supporting your new drug development.
Capable of accommodating small molecule drugs containing hydroxyl, amino, phenolic hydroxyl, and other functional groups, we design and synthesize linker-payload conjugates tailored to different drug chemical properties. We offer multiple conjugation strategies that precisely connect self-immolative spacers (such as PABC, DMED) with diverse payloads, achieving efficient conjugation between linkers and small molecule drugs to ensure controllable and specific subsequent drug release.
We tailor β-glucuronide linkers according to client project requirements, supporting personalized adaptation to various antibodies and diverse payloads, meeting the stability and release mechanism demands of complex ADC designs.
Expertise in glycosidic bond construction ensures β-configuration structures possess excellent enzyme recognition ability. We are proficient in synthesizing and modifying self-immolative spacers, satisfying complex linker design needs.
We excel at formulating efficient conjugation strategies for small molecule drugs containing hydroxyl, amino, phenolic hydroxyl, and other multifunctional groups. With extensive experience in ADC drug model adaptation, we ensure smooth conjugation processes.
Leveraging advanced synthetic processes and strict quality control systems, we guarantee each batch of linkers features high purity, structural integrity, and excellent plasma stability, providing reliable support for subsequent conjugation.
BOC Sciences is equipped with world-class analytical instruments capable of precise detection of complex linker structures, delivering comprehensive evaluation reports on purity, stability, and cleavage performance to ensure product quality.
Supported by a well-established pre-GMP production environment, we provide process development and batch production services from milligram to kilogram scale, helping clients smoothly advance preclinical and clinical research.
With efficient project management and mature synthesis platforms, BOC Sciences accelerates progress from design to product delivery, effectively shortening R&D timelines and helping clients seize market opportunities.
In addition to custom linker services, we offer integrated ADC technology solutions covering antibody modification, conjugation strategy development, and payload performance optimization, supporting clients in efficiently advancing ADC projects.

At the project's outset, BOC Sciences engages deeply with clients to fully understand antibody types, payload characteristics, and target DAR. Combining clients' R&D goals and technical challenges, we scientifically assess linker design feasibility and develop optimal customized plans, laying a solid foundation for subsequent development.
Our scientific team uses extensive databases and advanced design tools to precisely determine glycosidic bond construction methods, select self-immolative spacers, and formulate payload conjugation strategies. This ensures the linker's high plasma stability and efficient cleavage release, maximizing ADC performance.
In the small-scale phase, BOC Sciences optimizes reaction conditions and synthesis routes to achieve high yield and purity of linkers. We emphasize process simplification and green chemistry to ensure sustainability and cost reduction, providing reliable data support for scale-up.
Using advanced analytical platforms (NMR, MS, HPLC, etc.), we conduct comprehensive structural confirmation, purity testing, and stability evaluation. Each batch is accompanied by complete quality reports to ensure linkers meet stringent conjugation and preclinical research standards.
BOC Sciences offers production capabilities from milligrams to kilograms, supporting different stages of preclinical and clinical trials. Process scale-up and validation can be performed under pre-GMP conditions, fully preparing for large-scale manufacturing and ensuring smooth project progression.

Final delivery includes high-quality linkers, detailed synthetic routes, analytical data, and quality reports. We also provide ongoing technical consultation and support to assist clients in antibody conjugation and in vitro/in vivo evaluation, accelerating ADC projects into clinical development.
A glucuronide linker incorporates a hydrophilic sugar moiety cleaved by the lysosomal enzyme beta-glucuronidase. Once the sugar is removed from the phenolic backbone, self-immolation of the PAB group releases the free drug. Initially, this linker was used to conjugate MMAE, MMAF, and doxorubicin propyloxazoline to various antibodies for ADC formation. In later studies comparing glucuronide- and Val-Cit-PAB-linked ADCs, glucuronide-linked conjugates showed minimal aggregation (<5%) versus up to 80% in dipeptide-linked ADCs. Though in vitro efficacy was similar, the glucuronide linker demonstrated greater in vivo efficacy but had lower tolerability compared to Val-Cit-PAB.
β-Glucuronide linkers exhibit excellent plasma stability in ADC design, preventing premature drug release and systemic toxicity. They exploit the tumor microenvironment's enriched β-glucuronidase for specific, rapid drug release, enhancing therapeutic effects. Their simple, flexible structures make them a current focus in ADC research.
BOC Sciences offers comprehensive services from linker structural design, custom chemical synthesis, process development, scale-up, to multi-dimensional quality control. We optimize linker design based on antibody structure and payload properties to enhance conjugation efficiency and plasma stability, meeting strict standards for preclinical research and drug development.
Linkers typically covalently couple via self-immolative spacers (e.g., PABC) to reactive payload functional groups such as amines, hydroxyls, or phenolic hydroxyls. This design ensures that upon enzymatic cleavage by β-glucuronidase, the linker spontaneously cleaves, releasing active drugs while preserving payload activity and conjugation stability.
These linkers are well-suited for payloads containing hydroxyl, amino, and phenolic hydroxyl groups, commonly including MMAE, MMAF, doxorubicin, camptothecin, and other small molecule toxins. Rational linker design enables efficient, stable conjugation and enzyme-triggered release.
Selecting a linker structure requires considering antibody type, payload chemistry, and target environment. Design is based on payload functional groups, spacer length, and enzymatic sensitivity to balance plasma stability and rapid intracellular release, optimizing ADC performance.
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










