Disulfide linker is a key chemical component in antibody-drug conjugates (ADCs) and various targeted drug delivery systems. It is formed by two sulfur atoms linked through a disulfide bond, which remains stable under physiological conditions but enables precise drug release via disulfide linker cleavage in highly reductive environments such as tumor cells. This cleavable disulfide linker combines stability with controllable release properties, making it widely applied in tumor therapy, protein drugs, and nucleic acid drug delivery fields. BOC Sciences possesses profound expertise in organic synthesis and bioconjugation technologies, offering comprehensive customized disulfide linker development services. These cover linker structure design, synthesis and modification, disulfide cross-linking conjugation, release rate optimization, and thorough quality control. Whether for ADC disulfide linkers, cyclic disulfide linkers, or disulfide PEG linkers, we provide one-stop solutions from laboratory scale to industrial production, supporting efficient advancement of your drug development.
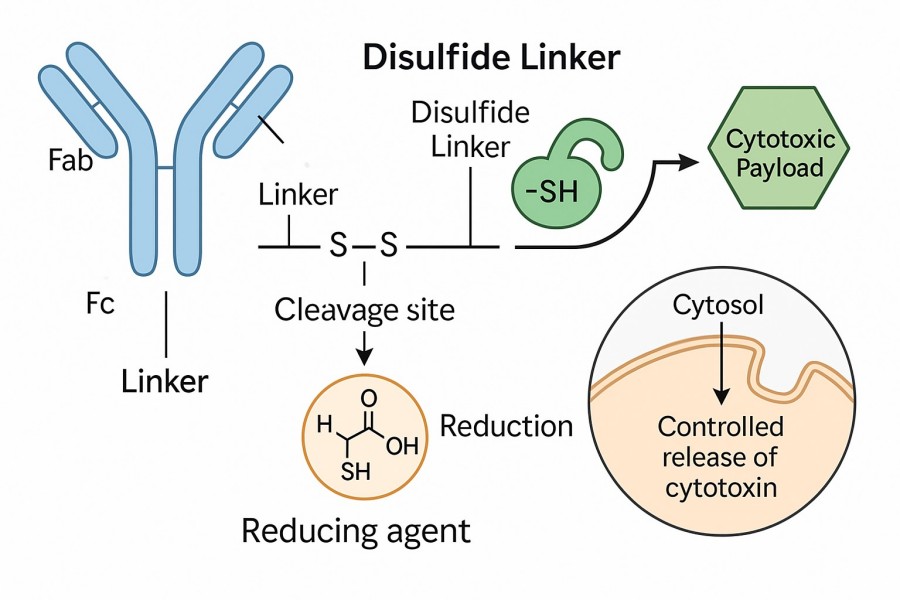
A disulfide linker (GSH-sensitive linker) is a type of chemical linker containing a disulfide (-S-S-) structure, widely used in ADCs and other targeted drug delivery systems. Its core chemical feature is the disulfide bond linker, formed by two sulfur atoms through disulfide cross-links, creating a stable yet cleavable structure. In ADCs, antibody chains linked by disulfide bonds remain stable in blood circulation. However, upon entering the reductive environment of tumor cells, cleavable disulfide linkers undergo disulfide linker cleavage triggered by reducing agents such as glutathione (GSH), releasing cytotoxic drugs to achieve precise tumor cell killing. Common types of disulfide linkers include cyclic disulfide linkers, disulfide PEG linkers, and cysteine disulfide cross-linking. In drug delivery, disulfide linkers are extensively used due to their controllable release and high targeting ability, not only in ADCs but also in siRNA, protein drugs, and small-molecule chemotherapy carriers.
BOC Sciences has over 15 years of experience in biochemical and medicinal chemistry synthesis, providing end-to-end disulfide linker synthesis services. Our expertise encompasses every stage of the development process—from initial molecular design and structural optimization to scalable synthesis, functional modification, and rigorous quality control. Leveraging state-of-the-art organic synthesis techniques and deep knowledge of bioconjugation chemistry, we collaborate closely with clients to create customized disulfide linkers that balance stability, cleavability, and biocompatibility.
In the development of ADCs and other targeted drug delivery systems, disulfide linker structure design must be compatible with specific antibodies and effective payloads. BOC Sciences offers customized solutions with high matching degree, high stability, and adjustable cleavability. We focus not only on chemical compatibility between linkers and drug molecules but also deeply consider antibody structure, payload properties, and pharmacokinetics to ensure optimal therapeutic efficacy and safety for every project.
We design highly compatible disulfide bond linkers for various antibody types (monoclonal antibodies, bispecific antibodies, antibody fragments) to ensure conjugation efficiency and antibody activity.
Provide dedicated disulfide linker drug delivery solutions for various payloads (small molecule toxins, nucleic acid drugs, proteins, nanoparticles) to achieve optimal release efficiency and safety.
Tailored disulfide bond linker solutions based on client drug characteristics and project goals, covering linker length, hydrophilicity, and cleavability, ensuring high compatibility with drug activity.
Offering cleavable disulfide linkers, cyclic disulfide linkers, PEG-modified and multifunctional cross-linking linkers to meet ADC, protein drug, and nucleic acid drug delivery requirements.
Composed of experienced chemists and biochemists proficient in cysteine disulfide cross-linking and complex conjugation strategies, ensuring high reaction efficiency and product purity.
Multi-dimensional testing with LC-MS, HPLC, NMR to comprehensively evaluate disulfide linker stability and purity, ensuring batch-to-batch consistency and reproducibility.
Efficient handling from milligram research scale to kilogram production, shortening disulfide linker synthesis cycles and supporting rapid advancement of ADC and drug delivery projects.
Providing a complete solution from linker design, synthesis, optimization to disulfide link antibody conjugation and release performance evaluation, reducing outsourcing and improving R&D efficiency.

Discuss project goals, drug types, and applicable disulfide linker mechanisms with clients in detail. Clarify linker length, hydrophilicity, cleavability, and other requirements. Our scientific team assesses feasibility and proposes initial design directions.
Design multiple disulfide bond linker schemes based on drug properties and targeting needs, including cleavable and cyclic disulfide linkers, screening for stability and release rate.
Perform laboratory-scale linker synthesis; test disulfide linker stability, purity, and reactivity; verify disulfide linker cleavage effects through cellular or in vitro experiments.
Optimize synthetic routes to improve yield and purification efficiency, ensuring quality consistency during scale-up. Applicable for disulfide linker synthesis from milligrams to kilograms.
Conjugate optimized linkers with antibodies or drugs via disulfide links or cysteine disulfide cross-linking, precisely controlling drug loading ratio and cross-linking sites.

Analyze linker stability, purity, and functionality using LC-MS, HPLC, NMR, ensuring delivered products meet all technical requirements with detailed analytical reports.
Reducible or glutathione-sensitive disulfide linker is an alternative to acid-labile hydrazone linker in ADC design. Disulfide linker-based ADC strategies rely on higher concentrations of reducing molecules such as glutathione in the cytoplasm (1-10 mmol/L) compared to the extracellular environment (~5 µmol/L in blood). Mechanistically, disulfide bonds resist reductive cleavage in circulation by being embedded in the linker. After internalization, disulfide bonds are reductively cleaved by abundant intracellular glutathione to release free payload molecules. Additionally, methyl is usually introduced next to the disulfide bond to further improve the circulation stability. Currently, this class of linker has been employed in Mylotarg® and several maytansine-based candidates in clinical trials. In short, disulfide bond linkers keep conjugates intact during systemic circulation, and are selectively cleaved by the high intracellular concentration of glutathione, releasing the active drugs at the tumor sites from the non-toxic prodrugs. Studies have shown that reduced glutathione presented in tumor cells' cytoplasma is up to 1000-fold higher than that present in normal cells' cytoplasma, and the tumor cells also contain enzymes of the protein disulfide isomerase family, which may contribute to reduction of the disulfide bond in cellular compartments. Furthermore, the reactivity of the disulfide bond can be modulated by steric hindrance. For example, the linker structure of SAR-3419 was fine-tuned for optimal antitumoral activity selecting SPDB-DM4, which displays a gem-dimethyl substitution on the payload side.
Representative disulfide linker-based conjugates contain the cytotoxic maytansinoids conjugated to the different monoclonal antibodies. In particular, huC242-SPDB-DM4 (IMGN242) is a novel ADC comprised of huC242 antibody conjugated to the potent maytansinoid via the cleavable disulfide-linker, which allows targeted delivery to pancreatic tumor cells and releases the potent maytansinoid in tumor cells. Compared with uncleavable huC242-SMCC-DM1 containing a thioether linker, huC242-SPDB-DM4 with an average of three to four maytansinoid molecules showed the approximate activities in vitro. However, huC242-SPDB-DM4 exhibited significantly higher activity in multiple xenograft tumor models in vivo. The conjugate, which was linked via a disulfide bond exert an excellent effect and clearance rate for the conjugate, was about four times faster than that for the antibody component.
Disulfide Linkers are unique in their cleavable disulfide linker properties, which enable precise drug release via disulfide linker cleavage in highly reductive environments such as tumor cells, minimizing toxicity to normal cells. At the same time, these linkers exhibit good disulfide linker stability in blood circulation, ensuring stable drug transport and enhancing the therapeutic effects of ADCs and targeted drug delivery systems.
We customize various disulfide bond linkers according to project needs, including cleavable disulfide linkers, cyclic disulfide linkers, and disulfide PEG linkers. In addition, we design special functionalized linkers supporting cysteine disulfide cross-linking and multi-site cross-linking, meeting application requirements across ADCs, protein drugs, nucleic acid drugs, and other fields.
We optimize disulfide linker stability by modulating the electronic effects and spatial structure around the sulfur atoms, including introducing steric hindrance groups, designing cyclic disulfide linker ring structures, and applying hydrophilic or hydrophobic modifications at both linker ends. These strategies ensure that the linker remains cleavable in reductive environments while prolonging stability during blood circulation.
Yes, we not only offer professional disulfide linker synthesis services but also complete antibody conjugation via disulfide links, drug payload optimization, release rate evaluation, and quality testing. Clients can entrust us with the full process from linker design to ADC disulfide linker production, reducing outsourcing steps and greatly improving R&D efficiency and project timelines.
We provide disulfide bond linker production services compliant with GMP standards according to client requirements. All production steps strictly follow quality management systems and are supported by comprehensive analytical testing (LC-MS, HPLC, NMR, etc.) to ensure the linkers meet the high purity, stability, and batch consistency standards required by pharmaceutical companies and clinical trials.
Background
An international oncology drug developer planned to develop a novel antibody-drug conjugate (ADC) for treating refractory solid tumors. The project's critical technical bottleneck was linker selection: it needed to guarantee disulfide linker stability during blood circulation to prevent premature drug leakage, while ensuring efficient disulfide linker cleavage inside tumor cells for precise drug release. The commercially available linkers previously used showed obvious shortcomings in drug-antibody ratio (DAR) control, release rate stability, and batch consistency, causing significant efficacy fluctuations in preclinical studies. The project urgently required a cleavable disulfide linker combining both stability and controllable release properties.
How BOC Sciences Helped
BOC Sciences customized a disulfide bond linker with high steric hindrance protection tailored to the client's antibody structure and payload characteristics, using cysteine disulfide cross-linking for conjugation. The core optimizations included:
Implementation Process
1. Requirement Analysis and Design
2. Small-Scale Synthesis and Validation
3. Pilot-Scale Synthesis and Conjugation Optimization
4. Pharmacology and Toxicology Support
Key Results
BOC Sciences has been deeply committed to the design and synthesis of ADC linkers for many years, with its products and technical solutions validated and cited in numerous high-impact international publications. Its related services include the structural design of various linkers, studies on cleavage mechanisms, stability optimization, and evaluation of pharmacological release, fully demonstrating BOC Sciences' technical depth and academic influence in precise drug delivery and bioconjugation.

"We have consistently faced challenges balancing linker stability and release rate in our ADC projects. The cleavable disulfide linker customized by BOC Sciences remains stable in blood circulation while rapidly releasing the payload inside tumor cells. This not only improved efficacy but also significantly reduced off-target toxicity."
— Dr. Laura Mitchell, Principal Scientist (United States)
"BOC Sciences' expertise in cysteine disulfide cross-linking technology is impressive. Their team provided full support in a short time frame—from linker design and synthesis to conjugation optimization—with excellent product purity and batch-to-batch consistency."
— Prof. Markus Vogel, Head of Bioconjugation Research (Germany)
"We needed a cyclic disulfide linker tailored to a specific antibody that would enhance conjugation efficiency without compromising immunoreactivity. The solution provided by BOC Sciences precisely matched the antibody structure and improved pharmacokinetic properties through structural optimization. The overall collaboration was highly professional and efficient."
— Dr. Sofia Andersson, Senior Research Scientist (Sweden)
"The BOC Sciences team demonstrated exceptional technical capability in their customized disulfide linker drug delivery services. Whether it was chemical compatibility assessment or drug-to-linker ratio control, they met our stringent standards. Their quality control processes gave us great confidence in the final product's stability and safety."
— Dr. Thomas Lefevre, Lead Formulation Scientist (France)
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










