Antibody-drug conjugates (ADCs) have become an effective solution for cancer therapy and targeted drug delivery systems. The creation of ADCs requires a sophisticated approach because the carrier antibody determines both therapeutic effectiveness and specificity, which affects the entire treatment outcome. The paper analyzes carrier antibodies' essential roles in ADC development alongside the challenges faced during antibody selection and optimization strategies.
ADCs achieve "precise elimination" of cancer cells through their dual mechanism of monoclonal antibodies for target recognition and small-molecule drugs for potent cytotoxic action. The carrier antibody functions as the ADC's scaffold and plays a critical role in directing the drug to the intended site while preventing off-target toxicity and regulating drug metabolism. The performance of ADCs in therapy depends heavily on the characteristics of the chosen antibody.
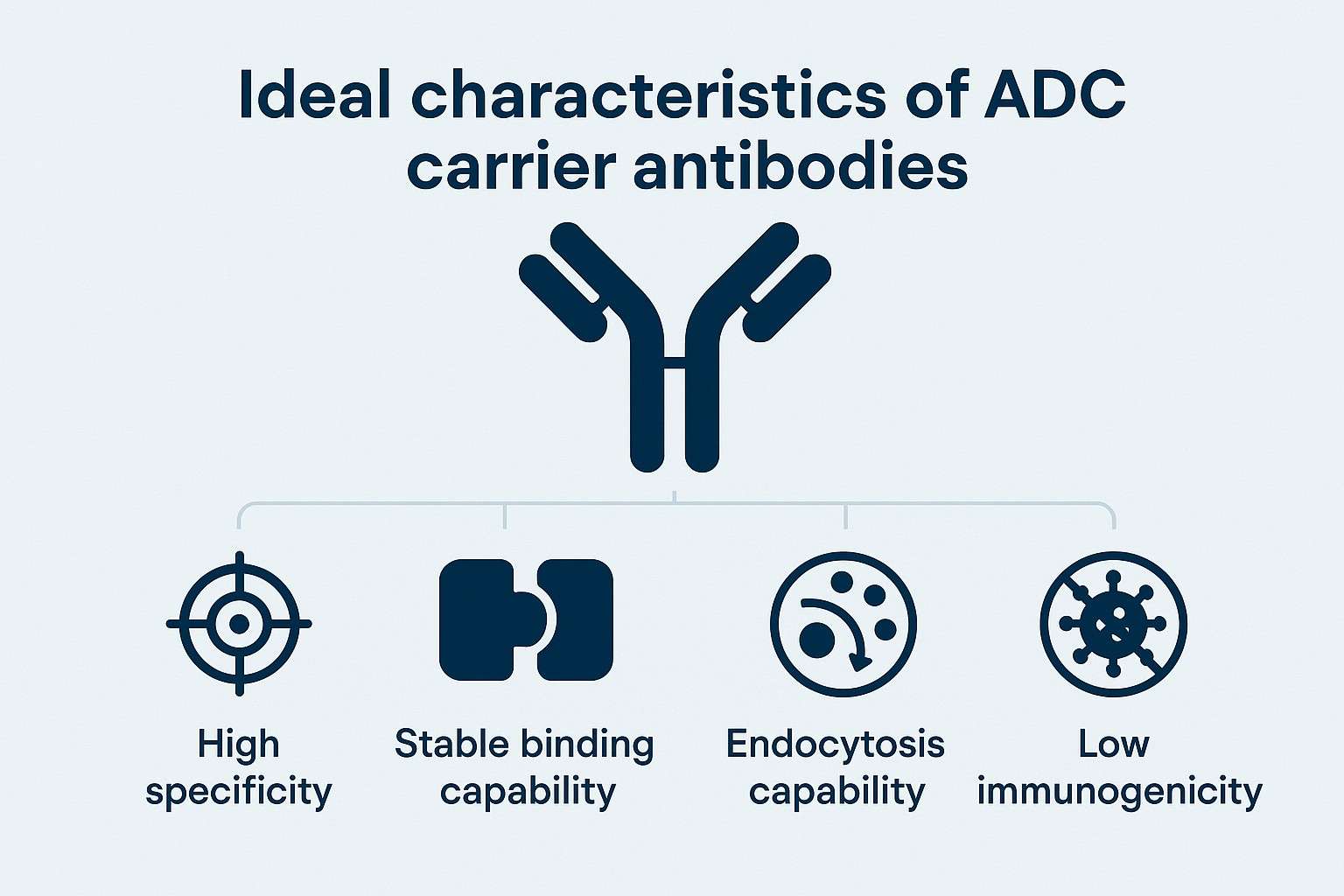 Fig. 1. Ideal characteristics of ADC carrier antibodies (BOC Sciences Authorized).
Fig. 1. Ideal characteristics of ADC carrier antibodies (BOC Sciences Authorized).
A carrier antibody refers to the monoclonal antibody used in an ADC as a "guidance tool," which specifically recognizes antigens on or inside tumor cells to ensure the cytotoxin exerts its effect only at the target site. Besides delivering the payload precisely, the carrier antibody also functions as a "sustained-release system," regulating the timing and distribution of drug release, thereby significantly expanding the therapeutic window. An ideal carrier antibody should possess the following characteristics:
The selection of carrier antibodies directly influences the targeting efficiency, toxicity control, drug release mechanism, and in vivo behavior of ADCs. Specifically, it affects:
For instance, an antibody with high affinity might still be ineffective if its target antigen lacks internalization capability, reducing the ADC's cytotoxic impact. Conversely, if the selected antigen is expressed on both tumor and healthy tissues, the ADC—even if efficiently conjugated—might exhibit intolerable off-target toxicity, hindering clinical progress. Therefore, ADC efficacy is not solely dictated by the payload but is the result of synergistic interactions among the antibody, linker, and payload—with the antibody being the most fundamental and decisive component.
The successful development of ADCs hinges on a critical prerequisite—selecting an ideal carrier antibody. However, identifying the most suitable antibody from a vast array of monoclonal options involves numerous technical and biological challenges. In the context of advancing precision medicine and multi-target strategies, balancing specificity, affinity, safety, efficacy, and manufacturability becomes a central challenge in carrier antibody development.
Choosing the right ADC carrier antibody is a formidable task primarily due to the complexity of antibody specificity and affinity. The target antigen must be selected based on its expression on cancer cells and minimal or absent expression in normal tissues. Yet even with a well-defined target, finding an antibody with both high specificity and optimal affinity is difficult. Antibodies may cross-react with structurally similar antigens, leading to off-target effects and potential toxicity. Moreover, the antibody's affinity for the target antigen must be finely tuned. Low affinity may result in weak binding and reduced efficacy, while overly high affinity could lead to rapid clearance from circulation or other unintended effects. Challenges can also arise from the antigen itself—some antigens may shed from the cell surface or exist in various isoforms, making effective binding harder to achieve. Antigen expression levels may also vary across cancer types and patients, further complicating antibody selection. Researchers must thoroughly assess both antigen and antibody properties to ensure that the carrier antibody functions as intended in the ADC system.
Another major challenge in carrier antibody selection is immune evasion. Cancer cells have evolved diverse mechanisms to escape immune detection, and these same strategies may impair ADC effectiveness. For example, some cancer cells may downregulate antigen expression or modify antigen structure to avoid recognition by the carrier antibody. To counter immune evasion, ADCs must be optimized to maximize their ability to reach and bind to target cells. This may involve selecting antibodies against antigens that are less likely to be downregulated or altered. Additionally, combination immunotherapies or multi-target strategies can be employed to enhance ADC efficacy. However, these approaches further complicate the antibody selection and optimization process.
Even if a carrier antibody demonstrates excellent targeting and therapeutic potential from a biological standpoint, its transition to practical production is constrained by multiple manufacturing and process development factors. First, compatibility with expression systems is a critical issue—some engineered antibodies may not be efficiently expressed in standard CHO cell lines, limiting scalability. Second, structural stability directly affects manufacturability. Antibodies prone to aggregation, denaturation, or fragmentation are unsuitable for downstream conjugation or long-term storage. Additionally, the distribution of conjugation sites (e.g., lysine or cysteine residues) significantly influences the homogeneity of the DAR, affecting consistency and quality control. Finally, considerations in clinical translation and industrialization include intellectual property clarity and compliance with GMP standards. Thus, comprehensive evaluation of manufacturability and process compatibility is an indispensable part of carrier antibody screening and optimization in ADC development.
| Conjugation Type | Service Description |
| Bispecific Antibody Conjugation | Enables dual-targeting ADCs by conjugating bispecific antibodies with precise control over payload attachment. |
| Carbohydrate Conjugation | Utilizes glycan moieties on antibodies for selective and mild conjugation to preserve antibody function. |
| Click Chemistry Conjugation | Employs bioorthogonal click reactions for rapid, efficient, and site-selective ADC construction. |
| Cysteine Conjugation | Targets reactive thiol groups on cysteines to achieve stable and efficient drug-linker attachment. |
| Enzymatic Conjugation | Uses enzyme-mediated reactions for site-specific and homogeneous ADC assembly. |
| Lysine Conjugation | Attaches payloads through accessible lysine residues for versatile and widely applicable ADC synthesis. |
| Non-natural Amino Acids Conjugation | Incorporates synthetic amino acids to introduce defined, orthogonal conjugation sites on antibodies. |
| Site-Specific Conjugation | Delivers precise control over conjugation sites to enhance ADC homogeneity and pharmacokinetics. |
| Tyrosine Conjugation | Leverages underutilized tyrosine residues for selective conjugation with minimal structural disruption. |
Faced with numerous challenges in the selection and design of carrier antibodies for ADC development, researchers and biopharmaceutical companies are continuously exploring effective solutions. Through multidimensional optimization strategies such as enhanced targeting, improved stability, and structural engineering, the overall performance of ADCs can be significantly improved—enhancing tumor targeting, prolonging in vivo half-life, and effectively reducing toxicity. Below are several common and critical antibody optimization pathways:
Screening monoclonal antibodies with high specificity and moderate affinity is the first step in constructing efficient ADCs. The core strategies include:
Modern technologies such as single-cell screening, antigen-antibody interaction databases, and human antibody libraries enable high-throughput identification of promising antibody candidates.
Naturally occurring antibodies often fail to meet the complex demands of industrial-scale ADC development, making antibody engineering a key optimization strategy:
Enhancing antibody circulation time and tumor tissue accumulation can significantly improve therapeutic efficacy and reduce dosing frequency. Common strategies include:
These approaches not only enhance the biological delivery capacity of ADC carrier antibodies but also extend their active window from a pharmacokinetic perspective.
In ADC drug development and translation, large-scale, consistent, and quality-controlled antibody production is a critical step for commercialization and sustainable clinical supply. Compared to general antibody products, ADC carrier antibodies face stricter requirements in quality, structural integrity, site-specific modifications, and impurity control. However, transitioning from lab research to industrial production poses several challenges, including cell line stability, yield fluctuation, purity control, and batch consistency.
Scaling from laboratory quantities (mg level) to pilot and commercial scales (g to kg level) presents technical difficulties not only in yield but also in maintaining structural, functional, and conjugation site consistency. Specific challenges include:
Addressing these issues requires repeated verification in cell engineering, culture condition optimization, and scale-up modeling, supported by precision online monitoring for closed-loop control.
The quality of ADC carrier antibodies is not just about expression yield but also their purity, homogeneity, and impurity profile—all critical for conjugation efficiency and final product stability. Main challenges in industrial preparation include:
To address these challenges, companies must establish robust downstream purification platforms with multi-step chromatography, membrane filtration, virus removal, and strict impurity control.
For large-scale antibody production used in ADC construction, compliance with GMP and comprehensive quality control and documentation are essential. Key tasks in establishing quality systems include:
As ADCs become a key approach in precision cancer therapy, the quality, specificity, and stability of carrier antibodies directly determine clinical outcomes. With years of experience in antibody engineering and integrated ADC development, BOC Sciences provides comprehensive and customized support for antibody selection, affinity tuning, structural engineering, and manufacturability to help global clients efficiently develop safe and effective ADC candidates.
From cytotoxins to linkers, explore our cutting-edge products for your ADC project.
| Catalog | Name | CAS | Price |
| BADC-00346 | Maytansine | 35846-53-8 | Inquiry |
| BADC-00033 | PNU-159682 | 202350-68-3 | Inquiry |
| BADC-01397 | Chimmitecan | 185425-25-6 | Inquiry |
| BADC-00800 | Exatecan Mesylate | 169869-90-3 | Inquiry |
| BADC-00324 | MMAE | 474645-27-7 | Inquiry |
| BADC-00086 | Mertansine | 139504-50-0 | Inquiry |
| BADC-00347 | DM4 | 796073-69-3 | Inquiry |
| BADC-01399 | Eribulin Mesylate | 441045-17-6 | Inquiry |
| BADC-00723 | N-Acetyl-Calicheamicin | 108212-76-6 | Inquiry |
| BADC-00341 | Seco-Duocarmycin SA | 152785-82-5 | Inquiry |
Explore our advanced tools and expertise for next-generation ADC research and development.
Evaluates ADC efficacy, stability, and cellular uptake through controlled laboratory assays.
Assesses pharmacokinetics, efficacy, and safety profiles in animal models to support preclinical development.
Maps the biodistribution of ADCs across tissues to optimize targeting and minimize off-target effects.
Quantifies the average number of drug molecules per antibody to ensure consistency and therapeutic performance.










