Enzyme-cleavable linkers are a class of chemical connecting units that can be cleaved under the action of specific enzymes. They are used to link active drug molecules with targeted carriers, such as antibodies, peptide chains, or nanocarriers, enabling precise drug release. At present, enzyme-cleavable linkers have become a particularly effective type of ADC linker due to their ability to selectively release payloads in the lysosomes of target cells. BOC Sciences focuses on the research and application of ADC linkers, offering end-to-end services from design and synthesis to functional validation. We are committed to providing flexible ADC enzyme-cleavable linkers at highly competitive prices. We provide global customers with the development of various ADC linkers and bioconjugation services, supporting clients to achieve key milestones in new drug development through a comprehensive and cutting-edge platform.
Enzymatically cleavable linkers have attracted significant attention in ADC development due to their superior plasma stability and release mechanisms. Typically, the advantage of using enzyme-cleavable linkers refers to their ability to selectively induce drug release at target cells rather than in circulation. Therefore, the only clinically explored enzyme-cleavable linkers are peptides sensitive to cleavage by lysosomal cathepsin proteases. Generally, linkers containing Val-Cit or Val-Ala sequences are most widely employed due to high stability in human plasma and efficient drug release toward the lysosomes of target cells. Moreover, a self-immolative para-aminobenzoyl carbamate (PABC) spacer is also required to ensure the cathepsin-mediated cleavage is unimpeded by the payload.
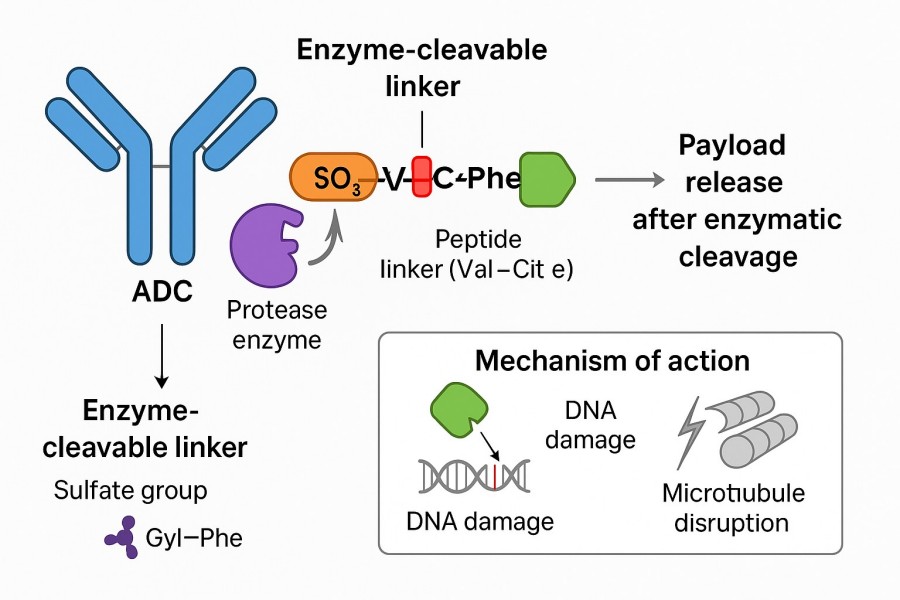 Fig. 1. Enzyme cleavable linker in ADC (BOC Sciences Authorized).
Fig. 1. Enzyme cleavable linker in ADC (BOC Sciences Authorized).
Enzymatically cleavable linkers provide antibody-drug conjugates with plasma stabilities comparable to non-cleavable linkers while boasting a more defined drug release method than disulfide-linked or acid-labile linkers. The most popular enzymatic cleavage sequence is the dipeptide valine-citrulline, combined with a self-immolative linker p-aminobenzyl alcohol (PAB). Mechanistically, cleavage of an amide-linked PAB triggers a 1,6-elimination of carbon dioxide as well as the concomitant release of free drugs in the parental amine form. In order to limit the release of payloads before internalization, preventing or minimizing degradation outside the target cell, the proteome of the lysosome became a logical place to search for enzymes capable of ADC degradation and present in high concentration.
BOC Sciences is committed to developing cutting-edge ADC technology. We have established a one-stop ADC technology development service platform, including mature ADC linkers development process, purification process, determining quality standards and key process parameters, as well as subsequent formulation development of products. BOC Sciences provides ADC linkers development service to customers worldwide to promote your ADC research projects.
Cathepsin B, a cysteine protease presents in the late endosome and lysosome compartments in mammals, is also overexpressed in many cancer cells. In fact, cleavable dipeptides were explored as cathepsin B substrates for doxorubicin prodrugs, demonstrating antigen-driven cellular activity with Val-Cit dipeptide linkers. BOC Sciences offers customized-design services for cathepsin B cleavable linkers according to the antibody, payload, and target. We design and select the most suitable linker based on the efficacy and toxicity of an individual ADC module to deliver high-quality and low-cost linker products in time.
Phosphatase is a hydrolase exhibiting selective expression in the lysosome. In 2016, researchers first designed phosphate and pyrophosphate-containing linkers coupled with the well-established, cathepsin B sensitive, Val-Cit-PABA moiety aiming to deliver glucocorticoids. Within studies, researchers demonstrated that efficient payload release is achieved by induction with lysosomal extracts, and both phosphate and pyrophosphate-containing ADC are active in vitro. BOC Sciences is experienced in supporting phosphatase cleavable linkers customization services for specific antibodies, payloads, and targets. We also offer optimized linker design schemes to balance ADC stability and payload release kinetics so that the release of payloads within tumor cells reaches its highest therapeutic threshold.
β-Glucuronidases are a class of glycosidase enzymes, which catalyze β-glucuronic residues hydrolysis. The abundance of β-glucuronidases in lysosomes and tumor interstitium is associated with the hydrophilicity of its substrates, which is also the reason of interest for designing safe and efficient cleavable linkers for ADC. A seminal work published in 2006 described the anti-CD70 ADCs releasing amine-containing MMAE, MMAF, and doxorubicin payloads, an original linker containing a β-glucuronic moiety attached to a self-immolative spacer. BOC Sciences offers customized-design services for β-glucuronidases cleavable linkers according to our client's project needs. We have established the most comprehensive ADC linker development services, including integrated linker design for dose scheme design and ADC efficacy.
β-Galactosidase is overexpressed in certain tumor tissues and cleaves lysosomal linkers via hydrolysis. Mechanistically, when the β-galactosidase cleavable linker was conjugated with trastuzumab and MMAE, it demonstrated higher potency than in conjugation with Val-Cit-PABC. Using the β-glucuronidase linker analogy, the cleavage mechanism involves the hydrolysis of the β-galactosidase moiety, which confers hydrophilicity to the chemical precursor. Another advantage is that the β-galactosidase enzyme is present only in the lysosome, whereas β-glucuronidase is expressed in lysosomes and also in the microenvironment of solid tumors. BOC Sciences aims to provide our global clients with β-galactosidase cleavable linker design services to support your most advanced research. Our highly skilled Ph.D. and M.S. synthetic chemists will solve the linker development challenges you encounter, from biological to chemical linker development.
Sulfatases offer an opportunity for selective payload release because they reserve high activity within the lysosomes but low activity in human and rodent plasma. There are various sulfatases that reside in the lysosome, catalyzing the hydrolysis of alkylsulfate esters into alcohols. Moreover, sulfatases are overexpressed in a number of different cancer types, thereby offering the possibility of additional selectivity for arylsulfate-containing ADCs towards tumors. BOC Sciences also provides customized-design services for enzyme-cleavable linkers to adjust ADC stability and payload release, and we are capable of constructing viable linkers and other ADC product developments at your request.
BOC Sciences possesses extensive experience and professional expertise in the design of enzyme-cleavable linkers, enabling the customization of efficient, stable, and controllable linkers for different drug molecules and carriers. Our design services cover everything from enzyme recognition site screening, chemical structure optimization, to conjugation strategy development, ensuring precise drug release within target tissues while minimizing systemic side effects. Through personalized design solutions, we help clients achieve efficient development of drug delivery systems. In drug development, our enzyme-cleavable linker design involves the following aspects:
Our experienced team of biochemistry and medicinal chemistry experts specializes in the design, optimization, and synthesis of various enzyme-cleavable linkers. We provide scientifically sound solutions tailored to different drug molecules and carriers, ensuring efficient and reliable project development.
Offer personalized design solutions based on specific project needs, including peptide sequence optimization, enzyme cleavage site selection, and conjugation strategy development, maximizing targeted drug delivery and precise release.
Provide complete one-stop services from molecular design, chemical synthesis, and drug conjugation to functional validation and stability evaluation, ensuring continuity and high efficiency throughout the linker development and application process.
By optimizing enzyme cleavage sites and linker structures, we achieve high stability of drugs in the bloodstream while ensuring release occurs only in target tissues or diseased cells, enhancing targeting and therapeutic safety.
Strictly follow GMP guidelines and quality control standards, ensuring traceability and reliability of each batch. We provide high-quality enzyme-cleavable linkers and related services to meet both research and commercial application needs.
Support applications in ADCs, nanocarriers, smart delivery systems, and diagnostic probes, meeting diverse biopharmaceutical R&D needs and enabling efficient integration of drug delivery and imaging.

Thoroughly understand the client's drug molecules, carrier types, and development goals. Evaluate the design requirements for enzyme-cleavable linkers to provide a scientific basis for subsequent design and ensure alignment with the overall project strategy.
Determine the optimal cleavage site and chemical structure based on target enzyme characteristics and drug properties. Optimize linker stability and controllable release capability while balancing blood circulation stability and targeting.
Prepare linkers using high-purity synthesis techniques and perform efficient conjugation with drugs and carriers, ensuring uniform drug loading, high conjugation efficiency, and molecular stability.
Conduct in vitro enzymatic cleavage experiments, drug release efficiency tests, and targeted release validation to ensure the linker achieves efficient and controllable drug release under designed conditions, meeting R&D requirements.
Evaluate the chemical stability and functional compatibility of the linker in blood, antibodies, or other carriers. Assess the impact of storage conditions and transport environments on product performance to ensure long-term reliability.

Provide comprehensive experimental data, analytical reports, and optimization recommendations to support iterative improvements. Offer professional technical guidance and continuous service for clients during drug development or production.
The enzyme cleavable linker mechanism refers to the mode of action of enzyme-cleavable linkers. When a carrier-drug conjugate enters target cells or tissues, specific enzymes recognize and cleave the linker, releasing the drug from the carrier. This mechanism ensures that the drug remains stable in the bloodstream and is released only at the target site, enhancing therapeutic precision and safety. It is a core principle for achieving controlled release in ADCs and smart delivery systems.
The primary function of enzyme cleavable linkers is to achieve targeted drug release and highly selective delivery. By recognizing specific cleavage sites, the linker remains stable in the bloodstream and releases the drug only within target tissues or cells. This improves therapeutic efficacy, reduces systemic side effects, and plays a key role in antibody-drug conjugates, nanocarriers, and smart delivery systems.
BOC Sciences offers a variety of enzyme cleavable linkers, including peptide-based linkers, glycosidase-cleavable, esterase-cleavable, and serine/cysteine protease-cleavable linkers. We also provide highly customized design solutions based on the client's drug molecules, carrier types, and application requirements, ensuring efficient cleavage in target tissues while maintaining linker stability and enhancing the precision and safety of drug delivery systems.
BOC Sciences designs enzyme cleavable linkers with chemical modifications, peptide sequence optimization, and structural improvements to ensure high stability in the bloodstream and non-target cells, effectively preventing premature drug release in non-target tissues. During the design phase, we also consider plasma protein compatibility and in vivo degradation pathways to ensure efficient drug release in target tissues, improving therapeutic outcomes while reducing systemic toxicity risk.
For the development of ADCs, we provide enzyme cleavable linker design services. By analyzing the properties of the targeting antibody and drug, we optimize the cleavage site and chemical structure to achieve efficient conjugation, specific cleavage, and precise drug release. This enhances ADC therapeutic selectivity and safety. Our services help clients accelerate development timelines while ensuring the stability and controllability of drug delivery systems.
We comprehensively validate linker performance through in vitro enzymatic cleavage experiments, drug release testing, and stability evaluation. Based on experimental data, we optimize chemical structures and conjugation strategies to ensure efficient drug release in target environments. By combining control of cleavage sensitivity with carrier compatibility design, we maximize drug targeting and therapeutic efficacy while minimizing systemic toxicity in non-target tissues.
Background
A German biopharmaceutical company focused on developing antibody-drug conjugates (ADCs) for HER2-positive breast cancer. In early studies, traditional esterase-cleavable linkers were used, but these showed insufficient in vivo stability and targeted release efficiency, causing premature drug release in the bloodstream and increasing systemic toxicity risk. The company sought BOC Sciences' professional support to optimize linker design and improve drug targeting and safety.
How BOC Sciences Helped
Key Results
Background
A biopharmaceutical company in Massachusetts, USA, was developing ADCs targeting colorectal cancer. The company aimed to utilize β-glucuronidase, which is highly expressed in tumor cells, for targeted drug release. However, the early use of non-specific linkers showed insufficient in vivo stability, with partial premature drug release in the bloodstream, limiting therapeutic doses and increasing systemic side effects. Therefore, they sought BOC Sciences' professional support to optimize the design of β-glucuronic acid linkers.
How BOC Sciences Helped
Key Results
The publications section of BOC Sciences showcases scientific results published by global clients using our products and services. These publications cover drug development, targeted delivery systems, and related applications, demonstrating the value and reliability of our technical support in high-level research.

"We needed a highly specific enzyme-cleavable linker for our ADC project. BOC Sciences provided a tailored design with excellent stability and high cleavage efficiency. Their technical support and prompt communication exceeded our expectations."
— Dr. Emily Carter, Senior Research Scientist (USA)
"BOC Sciences helped us optimize β-glucuronic acid linkers for targeted drug delivery. The process was smooth, and the functional validation data were comprehensive and reliable. They are a professional and dependable partner."
— Mr. Thomas Müller, Lead Scientist (USA)
"Facing challenges with in vitro and in vivo linker performance, BOC Sciences offered expert guidance on design and synthesis. Their team delivered high-quality enzyme-cleavable linkers with consistent batch quality."
— Dr. Claire Bennett, ADC Development Manager (UK)
"We required customized enzyme-cleavable linkers for preclinical ADC studies. BOC Sciences not only provided efficient synthesis but also detailed stability and cleavage reports, greatly accelerating our project timeline."
— Mr. Julien Dupont, Senior Biochemist (France)
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










