Cleavable linkers are a core component of antibody-drug conjugates (ADCs) and various bioconjugation systems, responsible for stably connecting antibodies, proteins, peptides, or small-molecule drugs to active payloads while enabling precise release in specific biological environments. With the advancement of precision medicine and targeted drug development, the design, chemical properties, and triggering mechanisms of cleavable linkers play a decisive role in the efficacy, safety, and therapeutic index of ADCs. BOC Sciences is dedicated to providing high-quality cleavable linker products and customized services to clients worldwide. We offer over 1,000 different types of linkers, including acid-sensitive, enzymatically cleavable, disulfide-based, and pH-sensitive linkers, catering to diverse research and development needs. All products comply with GMP standards, ensuring stability in circulation and precise release within the tumor microenvironment.
Comprehensive one-stop antibody-drug conjugate service platform
Large Stock
More than 1000+ high-purity products in inventory

Global Delivery
Warehouses in multiple cities to ensure fast delivery

mg to kg
Qualified facilities & equipment of cGMP laboratory

24/7 Technical Support
Strict process parameter control to ensure product quality

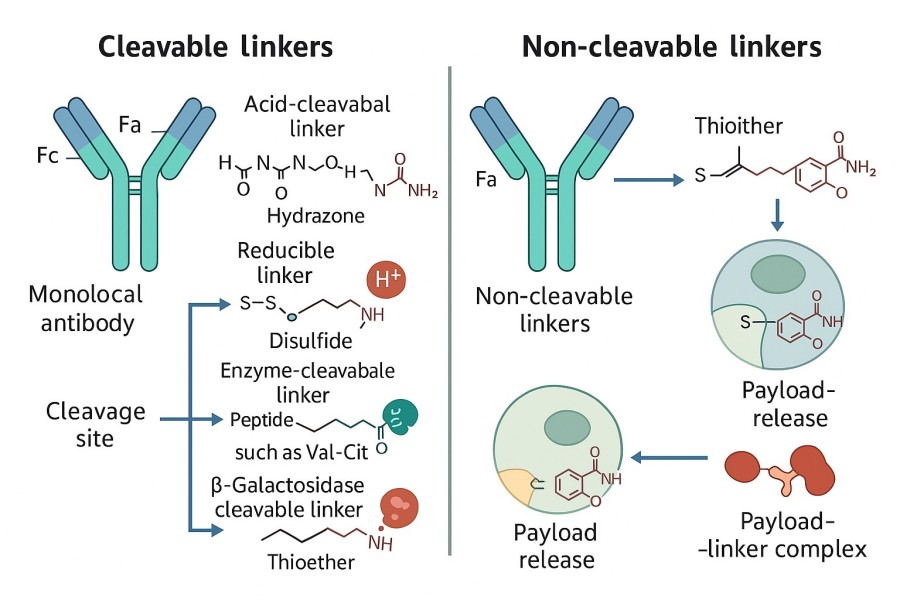
The ADC linkers significantly influence the timely release of the payload from the antibody. ADC linkers also determine the efficacy and adverse effects of a particular antibody. During drug discovery, an effective linker certainly increases the ADCs therapeutic index by ensuring the accurate release of the payload. Thus, linker construction is one of the most critical topics in ADC researches. In order to define suitable linkers for successful ADCs, the linkers must maintain high stability to ensure the integrity of ADCs in the blood circulation before reaching the tumor cells. Moreover, the ADC linker is responsible for the release of cytotoxins after ADC internalization. Besides stability, linkers also play a significant role in the physicochemical properties of ADCs. For example, most cytotoxic payloads are hydrophobic and linking these payloads to mAbs that contain additional hydrophobic properties usually cause ADC aggregation, which limits the drug loading content on antibodies. These linkers can be divided into cleavable linkers and non-cleavable linkers based on their dissociation properties.
Cleavable linkers are a core component in ADC design, connecting antibodies to cytotoxic drugs (payloads) through specific chemical or biological triggering mechanisms. Once inside tumor cells, cleavable linkers can respond to changes in the microenvironment—such as acidic conditions, enzymatic activity, or reductive environments—to achieve precise drug release. Compared to non-cleavable linkers, they significantly enhance the targeting and therapeutic efficacy of ADCs, making them a focal point in modern ADC development. Cleavable linkers are widely utilized in the ADC clinical pipeline, with acid-sensitive linkers like hydrazones and silyl ethers leading the way.
ADCs with cleavable linkers offer multiple advantages, optimizing drug delivery while increasing the therapeutic index and reducing the risk of side effects.

In ADC design, the choice of linker directly affects drug release efficiency, targeting, and safety. Cleavable and non-cleavable linkers each have their advantages and limitations. To help R&D teams and researchers better understand their different mechanisms and suitable applications in ADCs, a detailed comparison is provided across multiple key dimensions.
| Comparison Dimension | Cleavable Linkers | Non-Cleavable Linkers | Description |
| Drug Release Mechanism | Cleaved under specific conditions in the target microenvironment (e.g., acidic pH, enzymatic activity, or reducing conditions) for rapid payload release. | Payload release relies on antibody degradation or endosomal/lysosomal processing, slower release. | The release mechanism directly affects ADC efficacy and targeting precision. |
| Targeting Efficiency | High, allows precise payload release, minimizing damage to healthy cells. | Relatively lower, payload release is limited by antibody degradation rate. | High targeting efficiency improves ADC safety and therapeutic index. |
| Plasma Stability | Requires careful design to maintain stability in circulation; premature cleavage may occur | Generally high, stable in blood circulation, reducing off-target toxicity | Stability is a key factor for in vivo ADC performance |
| Suitable Payload Types | Compatible with a variety of drugs, especially cytotoxic small molecules needing rapid release. | Suitable for payloads that do not require rapid release. | Payload characteristics influence linker selection. |
| Clinical Application Strategy | Ideal for highly selective targeted therapy or tumors needing rapid drug action. | Better for sustained release or therapies requiring stable circulation. | Clinical application depends on pharmacokinetics and efficacy needs. |
| Antibody Structure Constraints | Requires flexible design to accommodate spatial constraints between antibody and payload. | Less structural restriction, can be directly attached. | Design must balance antibody structure and linker chemistry. |
| Risk of Side Effects | Targeted release reduces systemic toxicity, but premature cleavage may increase risk. | Lower systemic toxicity, but payload release at target may be insufficient. | Risk assessment is necessary during design. |
| Synthesis Complexity | Chemically complex, requires precise control over cleavage conditions. | Relatively simple synthesis, stability easier to control. | Synthesis difficulty affects production cost and process development. |
Cleavable linkers can be classified into various types based on their triggering mechanisms, each offering unique advantages in chemical structure, activation conditions, and application scenarios. Choosing the appropriate linker type is a critical step in ADC design, significantly impacting drug stability, targeting, and therapeutic efficacy. The main types include pH-sensitive, acid-cleavable, enzyme-cleavable, and disulfide-cleavable linkers.
pH-sensitive linkers respond to changes in environmental acidity or alkalinity. In acidic environments, such as the tumor microenvironment or intracellular endosomes/lysosomes, these linkers undergo chemical cleavage to release the payload. Common chemical designs include ester bonds or imidazole-derived bonds containing acid-sensitive linkages. Key features include:
pH-sensitive linkers have demonstrated excellent drug accumulation efficiency and controllable release in preclinical studies, making them a critical direction in modern ADC development.
Acid-cleavable linkers typically contain hydrolysis-prone bonds, such as hydrazones or acylhydrazones. In acidic environments, these bonds rapidly cleave, releasing the payload. Their main features include:
Acid-cleavable linkers are widely applied in preclinical ADC research and validated in several FDA-approved ADCs, providing a mature chemical strategy for drug design.
Enzyme-cleavable linkers utilize tumor-specific protease activity, such as Cathepsin B, Cathepsin L, or matrix metalloproteinases (MMPs), to release the payload. These linkers usually contain short peptide sequences recognizable and cleavable by enzymes. Key advantages include:
Enzyme-cleavable linkers are especially suitable for high-protease-activity tumor microenvironments, enhancing ADC therapeutic index and clinical safety.
Disulfide linkers rely on the intracellular reductive environment to trigger payload release. Tumor cells generally have elevated levels of glutathione (GSH), which can reduce disulfide bonds and release the drug. Key features include:
Designing disulfide linkers requires balancing circulation stability with targeted release efficiency, and chemical modifications can optimize pharmacokinetic properties for efficient and safe payload delivery.
BOC Sciences combines advanced chemistry and deep expertise to support your ADC linker development. From cleavable and non-cleavable linkers to tailored synthesis and complete ADC services, we deliver solutions designed for your project's success.
The core function of cleavable linkers is to release the payload under specific conditions, enabling efficient targeted therapy with ADCs. Understanding these mechanisms is essential for ADC design and optimization. Cleavable linker mechanisms primarily include chemical triggering, enzyme-catalyzed triggering, and reductive environment triggering.
The basic mechanism of payload release relies on controlled cleavage of the linker's chemical bonds. These mechanisms ensure that the payload acts only in the targeted microenvironment, minimizing non-specific toxicity to healthy tissues. Specific mechanisms include:
The tumor microenvironment presents multiple features that cleavable linkers can exploit for spatially and temporally precise drug release, ensuring rapid payload activity within target cells. Triggering conditions include:
From a chemical perspective, cleavable linker design depends on the controllable cleavage of specific bonds. Effective linker design balances circulation stability with targeted release efficiency. Stability in blood prevents premature systemic toxicity, while rapid cleavage in the tumor microenvironment ensures payload delivery to target cells.
The chemical design and structural optimization of cleavable linkers directly impact ADC performance, including circulation stability, targeting specificity, and payload release efficiency. Scientific chemical design principles and rational structural optimization are key to achieving high-performance ADCs.
The core of a cleavable linker lies in its scissile chemical bond, with different functional groups determining the linker's triggering mechanism and release properties. The selection and combination of these functional groups can be customized according to payload characteristics, tumor microenvironment features, and therapeutic strategy, enabling highly selective and efficient drug release. Key functional groups include:
Structural optimization is equally important when designing cleavable linkers. Fine-tuning structural parameters allows an optimal balance between stability, release efficiency, and selectivity. Key considerations include:
An effective cleavable linker achieves the optimal balance between stability, targeting specificity, and payload release efficiency. Scientific chemical design and structural optimization not only improve the ADC therapeutic index but also reduce the risk of adverse effects, providing a reliable foundation for clinical development.
Selecting the appropriate cleavable linker is critical to ADC development. The right linker ensures payload stability in circulation while enabling efficient release in the target microenvironment, maximizing therapeutic efficacy and minimizing side effects. Linker selection requires comprehensive consideration of payload characteristics, target tissue microenvironment, pharmacokinetics, and clinical requirements.
Careful evaluation of payload compatibility is the first step to ensuring ADC stability and efficacy. The chemical and physical properties of the payload directly influence the choice of cleavable linker:
Selecting cleavable linkers based on the target tissue microenvironment enables precise spatial and temporal payload release, improving therapeutic selectivity and safety. Tumor types and microenvironments vary significantly:
Pharmacokinetics (PK) and stability are critical factors in linker selection. Comprehensive evaluation of PK, stability, and clinical considerations helps R&D teams choose the optimal cleavable linker, ensuring maximal therapeutic effect while minimizing adverse reactions:
Cleavable linkers play a critical role in bioconjugation, enabling stable attachment of target molecules to active payloads while ensuring precise release under specific conditions. Their applications span ADCs, protein-drug conjugates (PDCs), peptide conjugates, and small molecule conjugates, providing powerful tools for modern drug development and biotechnology.
In ADCs, cleavable linkers connect antibodies to cytotoxic drugs. By leveraging acidic environments, enzymatic activity, or reductive microenvironments, the linker cleaves within tumor tissues to release the payload, achieving efficient targeted drug delivery. This strategy not only improves the therapeutic index but also significantly reduces toxicity to healthy cells, making it a core technology in modern ADC design.
In PDCs, cleavable linkers similarly connect proteins to small molecules or functionalized compounds. Proper linker design preserves protein structure while enabling payload release under specific intracellular or tissue-triggered conditions, allowing controlled drug release and functional protein modification. This strategy holds broad potential in the development of therapeutic protein drugs.
In peptide conjugates, cleavable linkers attach active payloads to functional peptides for precise targeted delivery. Peptides typically offer high selectivity and specificity, targeting specific receptors or tissues. Cleavable linkers release the payload under tumor microenvironment or endosomal/lysosomal conditions, enhancing drug accumulation and therapeutic efficiency. This approach is widely applied in targeted drug delivery, peptide functionalization, and fluorescent probe development.
In small molecule conjugates, cleavable linkers stably connect small molecule drugs or active compounds to carrier or targeting molecules. Triggered by pH, enzymatic activity, or reductive environments, the payload is released at the desired location, improving drug selectivity and bioavailability. This strategy is commonly used in targeted small molecule delivery, functional compound development, and bioconjugation molecule design, enhancing efficacy while precisely controlling release rates.
A cleavable linker is a key component in ADCs, connecting antibodies to cytotoxic payloads. Under specific conditions, such as acidic environments, enzymatic catalysis, or reductive microenvironments, the cleavable linker breaks, releasing the drug to enable targeted therapy while minimizing toxicity to healthy cells, improving ADC efficacy and safety.
The SMCC linker (Succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate) is typically non-cleavable. It forms a stable covalent bond between the antibody and payload and does not rely on microenvironmental triggers for release. Therefore, it is not considered a cleavable linker and is suitable for drug designs requiring prolonged circulation stability.
Common cleavable linkers include acid-cleavable linkers (e.g., hydrazone), pH-sensitive linkers, enzyme-cleavable linkers (peptide linkers, such as Cathepsin B-sensitive sequences), disulfide linkers, and redox-sensitive linkers triggered by reductive environments. All enable precise payload release.
A protease-cleavable linker is a type of linker that breaks under specific protease activity, often used in ADCs. By incorporating peptide sequences into the linker, cleavage occurs only in tumor cells or endosomal/lysosomal compartments with specific enzyme activity, enabling selective payload release, improving drug targeting, and reducing toxicity to healthy tissues.
Linkers for bioconjugation are used to covalently connect proteins, antibodies, nucleic acids, or small molecules. They typically contain reactive functional groups, such as amines, thiols, or carboxyls, which allow selective linkage between different molecules for targeted drug delivery, fluorescent labeling, or functional modifications. They are widely applied in ADCs, protein engineering, and biosensor development.
Chemically labile linkers are cleaved based on the differences in blood and cytoplasmic environments (pH, redox capacity, etc.). This mainly includes pH-sensitive linkers and disulfide bond linkers. Among all chemically labile linkers, hydrazones linkers are pH-sensitive and highly stable in blood neutral environment (pH 7.3~7.5). After ADCs internalization, hydrazone linkers beak and release cytotoxins due to the acidic environment. Disulfide bond linkers are another commonly used chemically labile linker. Since disulfide bond is thermodynamically stable, so its breaking depends on the concentration of intracellular glutathione. Usually, the concentration of the reduced glutathione in blood maintains at the micromolar level. However, since tumor cells are hypoxic, enhanced activity of reduced enzymes elevates the disulfide bond-breaking rate and releases cytotoxin.
Peptide linkers are the most widely used enzymatically cleavable linkers. Adcetris, an approved ADCs drug, conjugated microtubule inhibitor MMAE to monoclonal antibody cAC10 via valine-citrulline dipeptide linkers. Typically, valine-citrulline dipeptide linkers are highly stable in blood circulation. However, under a tumorous environment, elevated expression of lysosome protease B can enzymolyze the two amide bonds of the link and cause cytotoxins to release in the complete active form. The advantage of these linkers is that they not only maintain the ADCs' drug stability in blood circulation but also enable the rapid release of cytotoxins in the tumor site. Moreover, the release of drugs in an active structure without any additional modification maintains drug molecules' physicochemical properties and activity.
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.










