Antibody-drug conjugates (ADCs) achieve precise tumor cell killing by combining cytotoxic drugs (payloads) with specific antibodies. Among various payloads, DNA-damaging agents have attracted increasing attention from research institutions and pharmaceutical companies due to their high potency, unique mechanisms of action, and promising potential in treating solid tumors. With extensive experience in chemical synthesis and professional ADC development capabilities, BOC Sciences offers end-to-end services for DNA toxin-based ADC projects—ranging from molecular design, structural optimization, linker matching, ADC conjugation, and quality analysis to CMC support—helping clients advance their research efficiently and safely.
DNA-damaging agents are a class of active molecules that interfere with DNA structure and function, commonly serving as highly potent cytotoxic payloads in ADCs. These molecules achieve precise cell killing through multiple mechanisms, including double-strand breaks, single-strand cleavage, base alkylation, inter- or intra-strand crosslinking, and inhibition of DNA topoisomerase activity, thereby blocking DNA replication and transcription in cancer cells and inducing apoptosis. Common categories of DNA-damaging agents include alkylating agents, crosslinking agents, dna-cleaving agents, radioisotope payloads, and topoisomerase inhibitors.
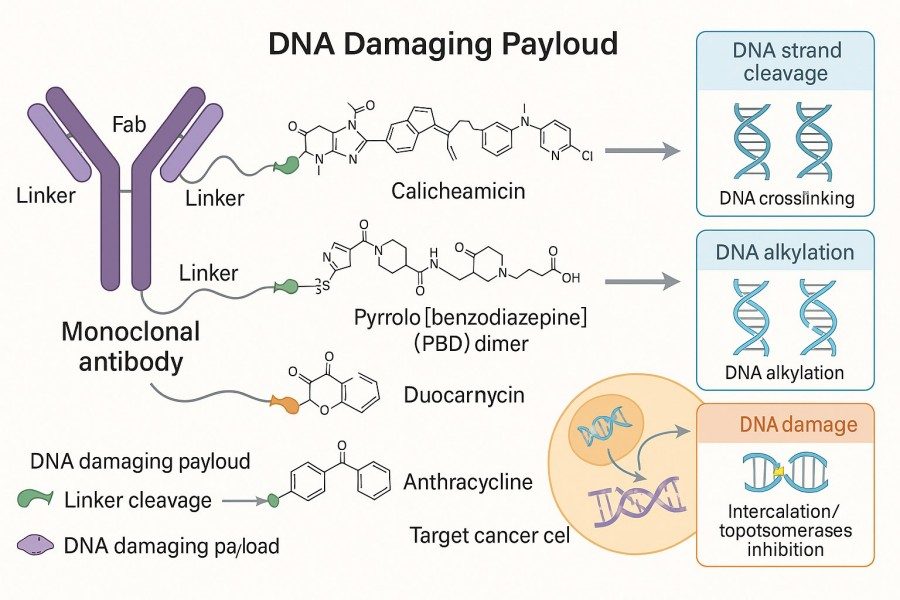 Fig. 1. DNA damaging agents in ADC (BOC Sciences Authorized).
Fig. 1. DNA damaging agents in ADC (BOC Sciences Authorized).
In ADC development, DNA-damaging agents are widely applied in targeted cancer therapy due to their potent and irreversible DNA-damaging properties. For example, PBD dimers and Calicheamicin-based payloads, which act via alkylation, have been successfully incorporated into multiple approved ADCs. Beyond ADCs, DNA-damaging agents are also used in chemotherapy drug development, tumor biology research, DNA repair mechanism studies, and high-throughput screening models, providing essential tool molecules for precision medicine and next-generation anticancer drug design.
Targets both highly proliferative and slowly proliferative tumor cells, applicable to both solid and hematologic tumors, thus expanding ADC applications.
Typical IC₅₀ values can reach picomolar (pM) levels; low doses can induce DNA damage, triggering cell cycle arrest and apoptosis.
Directly damages DNA through alkylation, strand breakage, or cross-linking, differing from tubulin-targeting toxins and less likely to cause drug resistance.
Certain DNA toxins possess membrane permeability, enabling them to diffuse and kill adjacent tumor cells that do not express the target antigen, thereby improving treatment efficacy.
Compared with traditional payloads, DNA toxins impose greater demands on R&D capabilities due to their structural complexity, synthetic challenges, handling safety, and conjugation strategies. Backed by over 20 years of small molecule custom synthesis experience and deep expertise in antibody conjugation, BOC Sciences has established a systematic DNA toxin development platform covering the entire process from structural design and linker screening to conjugation and scale-up. We are committed to providing global clients with high-quality, efficient, and translatable custom services, accelerating early-stage validation and clinical translation of ADCs, and solving technical and process challenges during DNA payload development.
BOC Sciences provides flexible, highly customized service modules tailored to client needs at different R&D stages, supporting critical steps such as payload screening, synthesis, structural optimization, linker matching, and ADC conjugation. We deeply understand the complexity of ADC development and specialize in collaborating with clients to build efficient workflows from molecular design to functional validation, accelerating project progress, reducing R&D risks, and achieving seamless transition from concept to clinic.
| Project Type | Client Scenario | Recommended Services |
| Lead Compound Screening | Clients exploring highly active payload candidates | Structural libraries + custom screening |
| Payload Optimization | Clients with existing structures needing solubility/stability improvement | Targeted modification and derivatization |
| Novel ADC Design | Clients with existing antibodies and targets | Linker selection + conjugation strategy support |
| Industrial Translation | Clients preparing for IND filing or process scale-up | Pilot-scale process optimization + quality system support |
From raw material procurement, structural design, and linker development to payload conjugation, ADC construction, and quality analysis, the entire workflow is managed in-house to ensure project continuity, data traceability, and efficient delivery.
A dedicated team of PhDs and technical experts in organic synthesis, ADC processes, and analytical testing, with rich experience in project management and cross-functional collaboration, ensuring both scientific rigor and execution excellence.
Equipped with advanced facilities and safety systems for handling highly toxic compounds, and experienced in working with ultra-potent toxins like PBD, Calicheamicin, and Duocarmycin, ensuring safe and compliant synthesis operations.
Supports both full-process, one-stop services and stand-alone development modules (e.g., toxin design, linker screening, ADC conjugation or characterization), tailored to meet specific project needs.
Enables smooth transition from research to clinical scale, with capabilities ranging from milligram-level synthesis to gram- and kilogram-level pilot production to match various R&D stages.
Advanced analytical platforms including LC-MS, HPLC, and SEC, combined with a comprehensive quality control system, support systematic evaluation of key parameters such as toxin purity, conjugation efficiency, and stability.

Based on the mechanism of action and project characteristics, we analyze suitable DNA toxin structures (e.g., PBD, Calicheamicin), design synthetic routes, and evaluate linker compatibility and release strategies.
Conduct lab-scale synthesis of small-molecule toxins, introduce functional groups, perform derivatization and linker docking experiments, and verify conjugation activity and basic stability.
Screen and optimize conjugation conditions between payload and antibody, including reaction type, solvent system, reaction time, DAR control, and purification methods.
Complete the conjugation of antibody and payload, use chromatography or membrane separation for purification, and ensure the homogeneity, stability, and bioactivity of the ADC product.
Conduct in vitro cytotoxicity analysis, endocytosis-release studies, and plasma stability assessments. Perform DAR, purity, and structural validation using LC-MS, HPLC, SEC, and other techniques.

Based on process feasibility verification, perform synthesis and conjugation scale-up. Support clients in transitioning from research to CMC stages, and provide batch samples with complete analytical data packages.
DNA damage can result from various factors, including endogenous sources such as reactive oxygen species, metabolic byproducts, and replication errors, as well as exogenous sources like radiation, ultraviolet light, chemical toxins, and certain drugs. In ADC development, understanding the mechanisms of DNA damage helps guide the design of DNA-damaging payloads to achieve targeted tumor cell killing.
Various toxins can damage DNA, including alkylating agents, interstrand crosslinkers, topoisomerase inhibitors, and certain natural toxins such as the carcinogenic aflatoxins. In the ADC field, these molecules are typically used as DNA-damaging payloads to target cancer cells.
We can support custom development of various DNA-damaging payloads, including but not limited to PBDs, Calicheamicin derivatives, Duocarmycins, Camptothecin derivatives (e.g., SN-38), and other DNA cross-linking or strand-breaking toxins.
Yes. We offer modular services, and clients can choose to engage only in payload synthesis, linker development, toxin optimization, or other individual service modules based on their project stage.
Yes. We have dedicated synthesis zones for highly active compounds, equipped with isolation ventilation systems, safety cabinets, and standardized operating procedures to strictly control personnel exposure and cross-contamination risks.
Absolutely. We have capabilities in structure optimization and novel molecule design. We can perform customized modifications based on client-provided lead structures or mechanisms of action to develop toxins with enhanced selectivity or potency.
We can provide payloads in "linker-toxin" format or as "naked toxins" according to client needs. We assist in matching suitable cleavable or non-cleavable linkers and perform conjugation verification.
We apply various analytical techniques such as HPLC, LC-MS, and NMR to conduct systematic analysis of toxin structure, purity, functional group integrity, and conjugation activity, ensuring each batch meets R&D or regulatory standards.
Yes. We provide full toxin-antibody conjugation services, including reaction optimization, DAR control, purification, and characterization to ensure ADC product quality is stable and reproducible.
Yes. We have synthesis and scale-up capabilities ranging from milligram to kilogram levels, providing high-quality batch samples and scale-up process support for preclinical studies and early CMC work.
Yes. We can perform preliminary functional assessments such as in vitro cytotoxicity assays, endocytosis-release efficiency studies, and plasma stability evaluations to help clients assess ADC therapeutic potential.
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










