IgG is a glycoprotein, with each CH2 domain of the Fc fragment containing an N-glycosylation site at Asn297. This glycosylation site can serve as an attachment point for payloads. The long distance between the glycan and the Fab region reduces the risk of disrupting antigen-binding during antibody conjugation. In addition, the distinct chemical composition of glycans compared to the antibody polypeptide chain enables site-specific modification, making them suitable conjugation sites. BOC Sciences focuses on carbohydrate-based conjugation technology (glycan conjugation), leveraging the natural glycans in the antibody Fc region for precise modification to construct ADCs with high accuracy and low heterogeneity. We provide end-to-end carbohydrate conjugation services from design to industrial-scale production, meeting diverse needs from early-stage candidate development to preclinical manufacturing.
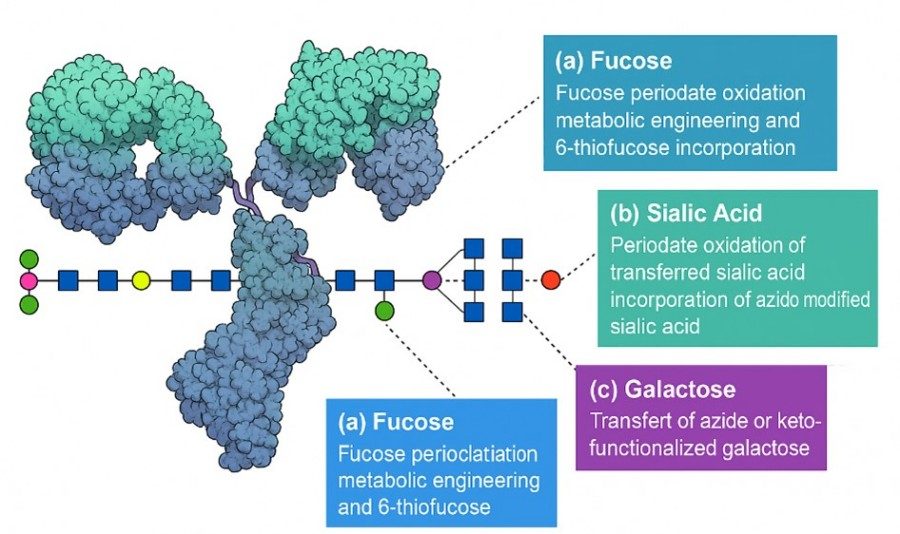
The antibody Fc region contains a conserved N-linked biantennary glycan at Asn297. Its spatial location is distant from the antigen-binding site, and its chemical composition differs from amino acids, making it amenable to site-specific modification. A common approach is mild oxidation of the glycan termini (e.g., sialic acids) to generate aldehyde groups, which can then react with derivatives containing hydrazide or aminooxy groups to form stable conjugation bonds (hydrazone or oxime). For example, Zhou et al. enzymatically added sialic acids via GalT and SalT, generating hydroxylated glycan termini that were oxidized with NaIO₄ to form aldehydes, then conjugated to drugs via oxime formation with aminooxy derivatives. Similarly, after removing galactose with β-galactosidase, engineered GalT can transfer galactose or N-acetylgalactosamine with terminal hydroxyl or azide groups to the glycan branches, followed by oxime or click chemistry to attach the payload. Additionally, endoglycosidases (e.g., EndoS/EndoS2 from Streptococcus) can trim the Fc glycan to the core GlcNAc, followed by "whole glycan transfer" with functionalized glycans or oligosaccharides onto the antibody for subsequent conjugation. In summary, these approaches share the principle of using the glycan as an anchor to introduce conjugation handles into the Fc glycan, enabling bioorthogonal reactions with the drug for site-specific conjugation.
Glycan conjugation enables precise, site-specific attachment to Fc glycans, producing ADCs with uniform drug distribution and predictable DAR. This reduces isomer formation, simplifies analysis, and ensures consistent quality across batches.
Fc glycans reside in concealed constant region clefts, away from antigen-binding sites. Conjugation here preserves antibody-target affinity and minimally impacts Fc-mediated functions such as FcRn binding and ADCC.
Shielded by surrounding amino acids, glycan conjugation uses mild reactions that reduce degradation or payload loss. Glycan modification also enhances ADC hydrophilicity, limits aggregation, and improves in vivo stability and safety.
Glycan conjugation requires no alteration of the antibody's primary sequence and can be applied directly to commercial antibodies. The method is simple, mild, cost-effective, and avoids complex protein engineering.
Leveraging extensive expertise in carbohydrate chemistry, enzyme engineering, and bioconjugation technologies, BOC Sciences has established a high-precision carbohydrate conjugation platform capable of achieving site-specific modification, highly homogeneous coupling, and controlled DAR construction under mild conditions. Our technical team is well versed in diverse antibody glycoforms, linker systems, and payload characteristics, enabling us to provide strategy design, process development, scale-up production, and comprehensive analytical support across all R&D stages. We assist clients in rapidly obtaining glycan-conjugated products with controlled structures, excellent stability, and strong suitability for downstream pharmaceutical development.
BOC Sciences specializes in site-specific conjugation through natural antibody glycans and glycan modifications, helping clients achieve ADCs with high homogeneity, stability, and functionality. Our services combine chemical and enzymatic approaches to flexibly accommodate different antibody types and payloads.
Classic glycan-based bioconjugation primarily targets the fucosylated N-glycan sites of IgG antibodies for site-specific modification. Fucose contains cis-diol structures suitable for selective oxidation. Using periodate oxidation or metabolic engineering to introduce thiol analogs generates aldehyde groups, which then react with hydrazide linkers to attach drugs. Compared to traditional cysteine conjugation, this strategy significantly reduces heterogeneity and produces ADCs with more predictable pharmacokinetic and pharmacodynamic profiles. BOC Sciences leverages this approach to provide stable and reliable conjugation solutions for drug development projects.
Antibody glycans can be enzymatically remodeled via galactosyltransferase and sialyltransferase to generate functionalized G2 glycans. Terminal galactose and sialic acids can then be oxidized to aldehydes and functionalized with hydroxylamine linkers. BOC Sciences performs enzymatic sialic acid addition followed by periodate oxidation or azide-functionalized sialic acid incorporation, producing conjugates with high in vitro targeting selectivity and excellent in vivo antitumor activity.
Galactose residues can serve as conjugation targets. After removing native galactose with β-1,4-galactosidase, engineered β-1,4-galactosyltransferase introduces galactose with keto or azide groups for bioorthogonal conjugation. BOC Sciences offers azide- or keto-modified galactose services, enabling ADC developers to achieve efficient, site-specific conjugation with improved homogeneity and functionality.
BOC Sciences uses IgG-specific endoglycosidases to enzymatically remodel antibodies, exposing core GlcNAc residues of natural glycans. Engineered GalT transfers UDP-GalNAz to the antibody, introducing an azide anchor for biocompatible, copper-free strain-promoted azide-alkyne cycloaddition (SPAAC) conjugation. ADCs produced via this GlycoConnect approach exhibit strong in vitro potency and excellent in vivo efficacy.
Conjugation using the natural glycans in the antibody Fc region allows precise control of modification sites, avoiding random modifications that may compromise antibody activity and improving ADC homogeneity and stability.
By finely tuning the number of conjugation sites on each antibody molecule, the drug–antibody ratio can be precisely controlled, optimizing efficacy and safety while minimizing off-target toxicity.
Glycan conjugation does not interfere with Fab-mediated antigen binding and can enhance Fc region conformational stability, ensuring high affinity and functionality of the antibody in both in vitro and in vivo experiments.
Compared to traditional cysteine or lysine conjugation, glycan conjugation significantly reduces structural heterogeneity of the conjugates, improving batch-to-batch consistency and predictability of pharmacokinetics.
Compatible with both chemical and enzymatic conjugation methods, glycan conjugation can be adapted to different antibody types and payloads, including small-molecule drugs, fluorescent probes, or nanocarriers, providing flexible solutions for diverse research needs.
Glycan conjugation forms stable hydrazone or bioorthogonal linkages, reducing non-specific drug release in vivo and enhancing ADC stability in circulation as well as antitumor activity.

Engage in in-depth discussions with clients regarding antibody type, payload, and application goals, assess feasibility, and develop a customized conjugation strategy to ensure a scientifically robust project plan.
Analyze the structure of antibody glycosylation sites and perform necessary glycan modifications or oxidation treatments to provide stable, site-specific reactive handles for subsequent conjugation.
Combine chemical and enzymatic conjugation methods to optimize reaction conditions and parameters, ensuring high conjugation efficiency, strong site selectivity, and preservation of antibody functionality.
Perform covalent conjugation of the antibody with drugs or labeling molecules under mild and controllable conditions, ensuring antigen-binding capability and structural stability are maintained.
Remove unreacted materials and assess DAR, homogeneity, and bioactivity using chromatography, mass spectrometry, HPLC, and SDS-PAGE, ensuring high-quality products.

Evaluate antigen-binding ability and in vitro activity of the antibody conjugates, providing a complete technical report and post-project support to ensure smooth progression and applicability of ADC projects.
Carbohydrate conjugation is a site-specific antibody modification technique that links small molecules, drugs, or functional groups to the antibody's sugar chains. By targeting natural glycosylation sites, this method preserves antigen-binding activity while achieving uniform DAR, improved stability, and reduced heterogeneity compared to traditional lysine or cysteine conjugation. It is widely used in ADC development, molecular imaging, and targeted delivery applications.
Carbohydrate conjugation is applicable to IgG, IgA, IgM, and various engineered antibodies, including humanized and chimeric antibodies. BOC Sciences can design site-specific conjugation strategies for Fc and Fab glycosylation sites of different antibodies. Glycan modifications can also be optimized based on antibody structure and application requirements to maximize conjugation efficiency and preserve antibody functionality.
Carbohydrate conjugation offers significant advantages in ADC development, including site-specific attachment, uniform DAR, preserved antibody function, and enhanced plasma stability. It reduces non-specific drug release, improves targeting selectivity, minimizes batch-to-batch variation, and optimizes pharmacokinetic and pharmacodynamic properties, enhancing both safety and efficacy in vivo.
BOC Sciences provides a complete carbohydrate conjugation service workflow, including project consultation and requirement assessment, antibody glycan analysis and preprocessing, conjugation strategy design and optimization, conjugation reaction execution, purification and quality control, and functional validation and project delivery. This workflow ensures high-efficiency, homogeneous antibody conjugates with preserved functionality, supported by technical reports and post-project assistance.
During glycan conjugation, BOC Sciences precisely controls glycan oxidation or functionalization reaction conditions to achieve controllable DAR. By combining chemical or enzymatic conjugation strategies, we can adjust the number of payloads per antibody molecule, ensuring uniformity, enhanced ADC efficacy, and in vivo stability, while minimizing non-specific release and toxicity risks.
Background
A European biopharmaceutical company is developing a next-generation ADC for hematologic malignancies. They aim to achieve higher conjugation site specificity and more stable DAR distribution to reduce impurity formation and enhance in vivo consistency. The team initially adopted lysine and cysteine conjugation approaches but found that:
Therefore, they shifted their focus to glycan conjugation to leverage the natural specific sites of Fc-region N-glycans, aiming to build a more homogeneous and controllable ADC. To achieve this goal, the company chose to work closely with BOC Sciences.
How BOC Sciences Helped
Based on extensive experience from multiple successful glycan site-specific conjugation projects, BOC Sciences performed glycan structural analysis, enzymatic modification assessment, and chemical reactivity testing on the client's antibody. A tailored enzymatic–chemical two-step glycan conjugation platform was ultimately designed for both the antibody and its payload. Through controlled glycan modification, specific reactive groups were introduced so that the conjugation site was strictly restricted to the N297 glycan region of the Fc domain, ensuring a highly homogeneous DAR structure. To enhance ADC solubility and stability, BOC Sciences also adjusted the hydrophilic/hydrophobic balance of the linker according to the payload's hydrophobicity, making the overall molecular properties better suited for in vivo conditions.
Implementation
Results
Browse BOC Sciences' publications to explore articles from research teams worldwide, showcasing the scientific contributions of our products and services in cutting-edge drug development.

"BOC Sciences gave our glycan conjugation program a clear competitive edge. Their precise site control and excellent batch consistency significantly accelerated our antibody optimization work."
— Dr. Michael Turner, Senior Protein Engineer (United States)
"As we advanced a new ADC design, BOC Sciences delivered highly professional carbohydrate conjugation support. Higher yields, lower impurities, and fast turnaround — a partner we truly trust."
— Ms. Anna Kovacs, Bioconjugation Scientist (Hungary)
"The glycan-modified antibodies from BOC Sciences were consistently high-quality with clean, reliable analytics. Stable, efficient, and dependable — they helped move our early screening projects forward much faster."
— Mr. Thomas Schneider, Antibody Discovery Lead (Germany)
"BOC Sciences demonstrated outstanding expertise in glycan-directed conjugation. The high-quality site-specific conjugates performed exactly as needed, giving us strong confidence for downstream development. A partner worth working with long-term."
— Dr. Isabelle Martin, Principal Research Scientist (France)
References
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










