Antibody-drug conjugates (ADCs) have emerged as one of the core innovations in modern oncology. As a critical component of precision drug delivery systems, the choice of drug payload directly influences the therapeutic efficacy and safety of ADCs. BOC Sciences has deeply engaged in ADC research and development, dedicated to providing comprehensive ADC payload development solutions to global clients, supporting the creation of next-generation ADC products with enhanced efficacy and safety.
In the construction of ADCs, the drug payload is the core element of the entire molecular structure. It not only directly determines the cytotoxic potential against tumor cells but also profoundly affects the pharmacokinetic properties, therapeutic index, and clinical safety of ADCs. The design concept of ADCs lies in guiding highly potent drugs selectively into tumor cells via monoclonal antibodies. As the "lethal blow" of this system, the drug payload is the key to achieving highly effective and precise therapy.
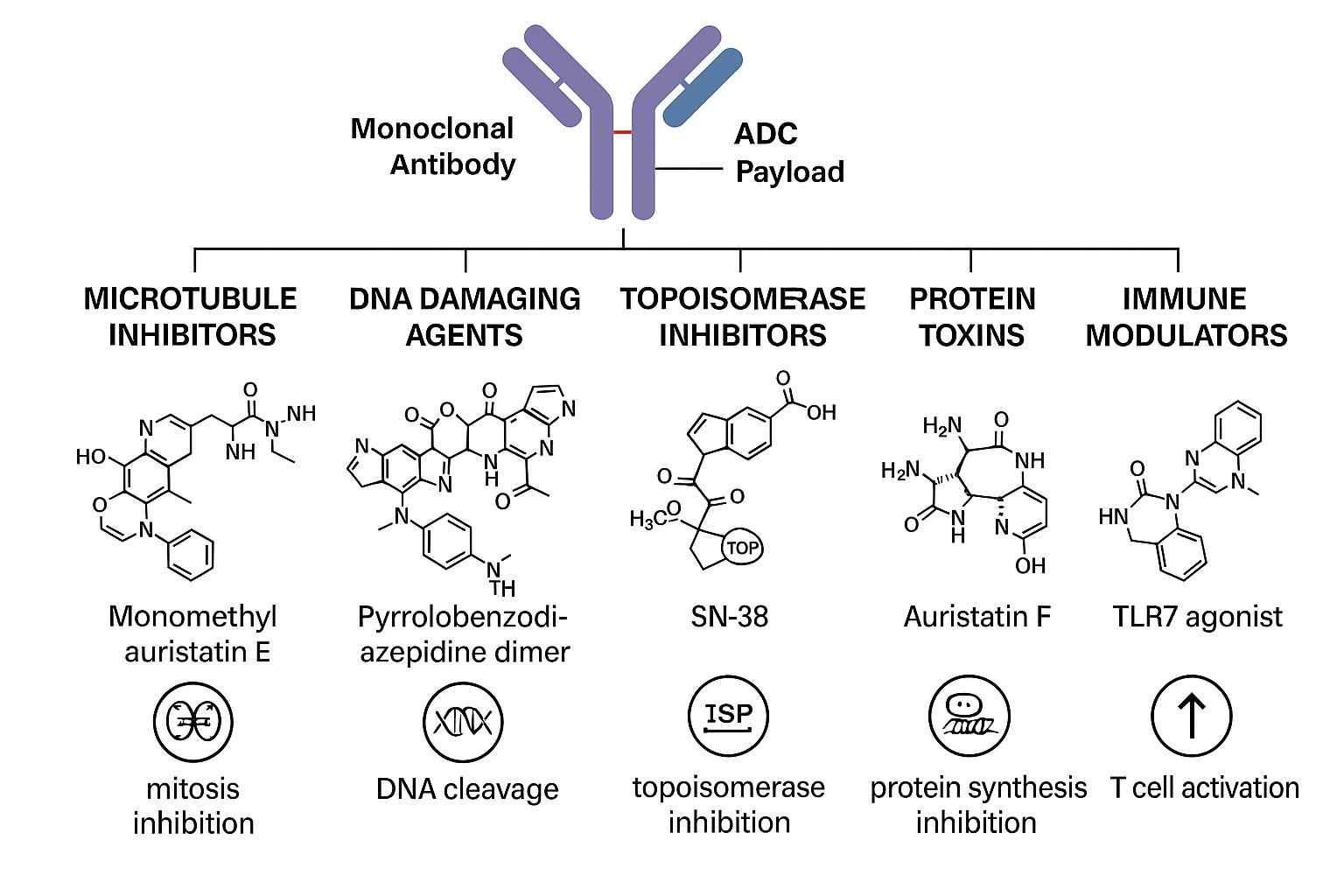 Fig. 1. ADC payload classes (BOC Sciences Authorized).
Fig. 1. ADC payload classes (BOC Sciences Authorized).
An ADC drug payload refers to a highly potent cytotoxic small molecule that is chemically conjugated to an antibody. Its purpose is to be released within tumor cells after antibody-mediated specific delivery, inducing cell death. To meet the unique requirements of ADCs, these payloads are generally designed with extreme cytotoxicity, ensuring that even minimal amounts released in tumor tissue can elicit strong antitumor effects. Currently, commonly used payload types include microtubule inhibitors, DNA-damaging agents, topoisomerase inhibitors, and emerging protein degraders, each working through different mechanisms to effectively eradicate tumor cells. The design of these molecules must balance in vivo stability, intracellular activity, and chemical compatibility with linkers to ensure superior therapeutic performance both in vitro and in vivo.
The drug payload is not only the direct "executor" of tumor cell death but also the core factor determining the clinical performance of ADCs. Its mechanism of action, intracellular localization, stability, and release profile all directly impact the therapeutic efficacy and safety of ADCs. The decisive influence of payloads on efficacy is reflected in several aspects:
Choosing an appropriate drug payload in ADC development requires integrating multiple factors to achieve the ideal "high efficacy + low toxicity" balance. Key considerations include:
In ADC development, selecting the drug payload is one of the most decisive yet challenging steps. Researchers must find the optimal balance between drug potency, toxicity, stability, and delivery selectivity to ensure the clinical safety and efficacy of ADCs. Each challenge involves complex issues in medicinal chemistry, pharmacokinetics, and tumor biology, requiring rigorous design and in-depth investigation.
The primary advantage of ADC drug payloads lies in their ultra-high cytotoxicity, enabling them to kill tumor cells at very low doses. However, this potency also poses significant toxicity risks, especially if payloads are prematurely released into the bloodstream. Early leakage of payloads in plasma can cause severe damage to high-metabolic tissues such as the liver and bone marrow, greatly limiting the therapeutic window and maximum tolerated dose of ADCs. Development teams must ensure sufficient antitumor activity while minimizing off-target toxicity to healthy tissues, necessitating payloads with excellent chemical stability and appropriate pharmacological properties.
The therapeutic concept of ADCs relies on antibodies guiding payloads precisely into tumor cells. However, tumor heterogeneity and complex microenvironments often make this process challenging. Variability in antigen expression among tumor cells can result in ineffective internalization of ADCs in some cells. Additionally, high interstitial pressure and dense extracellular matrices within tumors can impede ADC tissue penetration and distribution, affecting payload release efficiency. To complicate matters further, non-target cells, such as phagocytic cells in the liver, may nonspecifically uptake ADCs, leading to payload loss and reduced tumor accumulation and efficacy.
The method of linking drug payloads to antibodies and the stability of these conjugates largely determine ADC pharmacokinetics and clinical safety. If the payload-antibody conjugate is too unstable in circulation, premature payload release may lead to increased toxicity and reduced efficacy. Conversely, if the conjugate is overly stable, the payload may not be efficiently released within target cells, diminishing therapeutic effectiveness. Choosing appropriate linker types, optimizing payload chemical structures, and controlling conjugation site uniformity are critical strategies for enhancing ADC stability. These factors collectively influence every stage from in vivo distribution to intracellular release.
| Payload Type | Description |
| Auristatins | Highly potent microtubule inhibitors engineered for precision tumor targeting in your ADC development. |
| Calicheamicins | Ultra-potent DNA-cleaving agents to empower next-generation ADC payload design. |
| Camptothecins | Topoisomerase I inhibitors optimized for enhanced stability and tumor-specific delivery. |
| Daunorubicins/Doxorubicins | Proven anthracyclines tailored for ADC applications with controlled cytotoxicity. |
| Duocarmycins | DNA minor groove alkylators offering exceptional potency for targeted cancer therapeutics. |
| Maytansinoids | Tubulin-targeting cytotoxins ideal for ADCs requiring high efficacy and selectivity. |
| Pyrrolobenzodiazepines | DNA crosslinking agents designed to achieve superior anti-tumor activity in ADCs. |
| Traditional Cytotoxic Agents | A broad range of validated chemotherapeutics for versatile ADC payload solutions. |
The success of ADCs largely depends on the type of drug payload and its mechanism of action. Different payload types employ diverse strategies to kill tumor cells, including microtubule disruption, DNA damage, topoisomerase inhibition, and emerging protein degradation mechanisms. With advances in technology and medicinal chemistry, the range of payloads continues to expand, opening broader prospects for ADC applications.
Microtubule inhibitors are the most widely used class of ADC drug payloads. Their primary mechanism involves disrupting the assembly and disassembly of microtubules, thereby blocking mitosis and inducing tumor cell apoptosis. Representative molecules such as MMAE (monomethyl auristatin E) and DM1 (maytansinoid DM1) have been incorporated into several approved ADCs, including Adcetris and Kadcyla. The advantage of these payloads lies in their high selectivity for rapidly dividing tumor cells. However, their potent cytotoxicity also demands excellent plasma stability to prevent damage to non-target tissues.
DNA-damaging agents induce tumor cell death by causing DNA strand breaks or crosslinking, thereby blocking DNA replication and transcription. Calicheamicin and PBD (pyrrolobenzodiazepine) dimers are representative of this class. Calicheamicin has been applied in Mylotarg, while PBD dimers are utilized in ADCs such as Zynlonta. These payloads, with their extreme cytotoxicity, are suitable for treating tumors resistant to conventional chemotherapy. However, their clinical use faces challenges, including the risk of systemic toxicity from premature release, making precise linker design essential to ensure targeted delivery.
Protein degraders are an emerging class of payloads gaining considerable attention. These molecules work by inducing degradation of target proteins within tumor cells. PROTACs (proteolysis targeting chimeras) and molecular glues are the main focus of current research. They offer new therapeutic strategies for ADCs by addressing "undruggable" targets that traditional drugs cannot modulate. Although still in early stages of development, these payloads hold promise for overcoming tumor resistance and expanding ADC treatment options.
Topoisomerase inhibitors are increasingly studied as ADC payloads. They inhibit topoisomerase I or II, blocking DNA unwinding, which in turn impedes DNA replication and repair, leading to tumor cell death. SN-38, a potent topoisomerase I inhibitor, is among the most representative and has been incorporated into several ADC candidates in clinical trials. Compared to other payload types, topoisomerase inhibitors show potential efficacy against slower-proliferating tumor cells, broadening the therapeutic scope of ADCs.
In addition to conventional payloads, researchers are actively exploring novel payload types such as RNA interference agents, photosensitizers, and immunomodulatory molecules. These innovative payloads not only target tumor cell diversity but also enhance anti-tumor immune responses through combination therapies. With ongoing improvements in payload chemistry and targeting technologies, these advancements are expected to become key components of next-generation ADCs, providing new treatment solutions for refractory cancers.
Optimizing drug payloads is a critical strategy to improve the overall therapeutic performance of ADCs. Scientists continue to explore how to maintain potent tumor-killing activity while minimizing systemic toxicity and achieving precise treatment for different tumor types. The pharmacological properties, chemical structure of the payload, and its interaction with antibodies and linkers are central to design and optimization strategies.
Enhancing the potency of drug payloads is fundamental to achieving superior anti-tumor activity. However, this must not come at the cost of increased toxicity. One optimization strategy involves introducing specific hydrophilic groups or structural modifications to improve solubility and metabolic stability, thereby reducing non-specific tissue exposure. Additionally, modifying the mechanism of action or selecting multi-target cytotoxic molecules can enhance intracellular lethality while keeping effects on normal tissues within acceptable limits. Achieving this balance requires careful fine-tuning between chemical modifications and biological activity to ensure an optimal therapeutic index in both preclinical and clinical settings.
Precise release of payloads is critical for the efficacy and safety of ADCs. Premature release in circulation can result in severe systemic toxicity, while delayed release may lead to insufficient tumor cell killing. To address this, designing payloads with controlled release characteristics is essential. Strategies include developing pH-sensitive or reduction-sensitive structures tailored to tumor microenvironment conditions to trigger release only in target tissues. Additionally, regulating the chemical stability of linkers and payloads ensures prolonged stability in plasma while enabling efficient cleavage within tumor cells. These design principles allow ADCs to exploit tumor microenvironment differences for spatial and temporal precision in drug release.
Tumor heterogeneity dictates that different tumor types may exhibit significantly varied sensitivities to the same payload. Developing customized drug payloads for specific tumor types has become an important approach to improving clinical success rates. By thoroughly investigating the molecular characteristics, metabolic pathways, and resistance mechanisms of tumor cells, researchers can screen or design payloads that are highly compatible with these features. For instance, microtubule inhibitors may be more effective against rapidly proliferating solid tumors, while DNA-damaging agents could suit tumors with impaired DNA repair mechanisms. Furthermore, integrating tumor genomic data into payload development holds great promise for achieving highly precise, individualized treatment.
BOC Sciences is committed to providing global clients with one-stop, customized ADC drug payload development services. With a robust R&D platform and extensive industry experience, we help clients efficiently advance from payload design and screening to clinical-grade production. Our services encompass mainstream and innovative payload types, integrating advanced technologies to ensure that every project meets the highest standards of quality and efficacy. From early-stage payload screening to GMP manufacturing for clinical applications, BOC Sciences supports your ADC program, helping your products reach the market faster.
From cytotoxins to linkers, explore our cutting-edge products for your ADC project.
| Catalog | Name | CAS | Price |
| BADC-00340 | PBD dimer | 1222490-34-7 | Inquiry |
| BADC-00798 | Actinomycin D | 50-76-0 | Inquiry |
| BADC-00328 | Topotecan | 123948-87-8 | Inquiry |
| BADC-00325 | Paclitaxel | 33069-62-4 | Inquiry |
| BADC-00004 | Colchicine | 64-86-8 | Inquiry |
| BADC-00041 | Daunorubicin hydrochloride | 23541-50-6 | Inquiry |
| BADC-00253 | Luteolin 7-O-glucoside | 5373-11-5 | Inquiry |
| BADC-00361 | Combretastatin A4 | 117048-59-6 | Inquiry |
| BADC-00184 | Tubulysin A | 205304-86-5 | Inquiry |
| BADC-00038 | Doxorubicin hydrochloride | 25316-40-9 | Inquiry |
Explore our advanced tools and expertise for next-generation ADC research and development.
Biologic agents enabling highly specific and potent ADC therapies.
Small molecules designed for stable and effective ADC payloads.
Advanced nanocarriers improving drug delivery and tumor penetration efficiency.
Potent protein toxins for innovative and targeted ADC development.










