In the field of antibody-drug conjugate (ADC) development, lysine conjugation is one of the most classic, mature, and highly compatible drug-loading strategies, particularly suitable for rapid prototyping, early-stage development, and multi-drug screening. Leveraging years of ADC conjugation experience, BOC Sciences provides comprehensive lysine conjugation services, including customized payloads and linkers, reaction condition optimization, small-scale to scale-up preparation, purification, and multidimensional quality control analysis. By precisely controlling reaction kinetics, assessing site accessibility, and optimizing buffer systems, we achieve concentrated DAR distributions, maintain antibody activity, and ensure high batch-to-batch consistency. With complete QC reporting and technical support, BOC Sciences delivers high-quality ADC conjugation solutions that can be directly applied from early-stage research to later-stage production.
Lysine is an amino acid naturally found in proteins. It has a reactive amino group that can react chemically with drugs. By engineering the antibody to introduce lysine at specific positions on the antibody, a special catalyst is used to couple the payload to the native lysine to form an ADC. Currently, lysine coupling has been used in the production of three FDA-approved ADCs. A typical immunoglobulin G1 (IgG1) antibody has approximately 90 lysine residues. When targeting drug-to-antibody ratio (DAR) values of 2 to 4, the non-selective conjugation strategy has the potential to provide statistically up to 106 different isomers. In industry, process conditions are optimized and coupling occurs at 8-10 kinetically preferred sites of 90 lysines. This method has the following advantages:
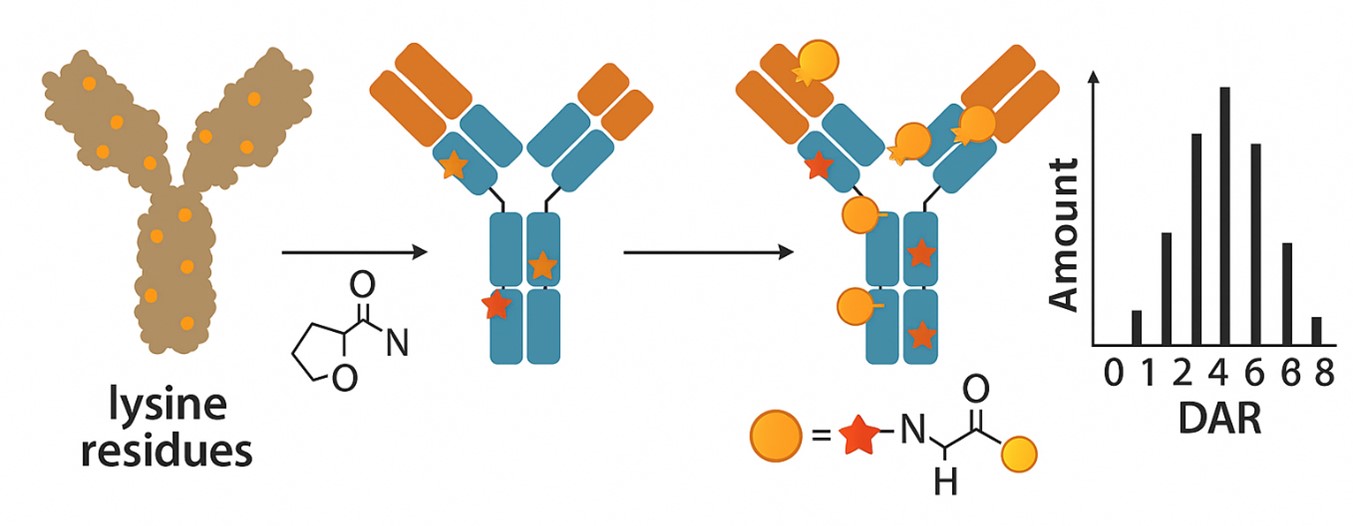 Fig. 1. Lysine conjugation in ADCs (BOC Sciences Authorized).
Fig. 1. Lysine conjugation in ADCs (BOC Sciences Authorized).
ADCs prepared using native lysine site-directed coupling technology have high stability and efficiency. Due to the stable carbon-carbon bonds formed between the antibody and the drug, ADC will not dissociate in the blood, ensuring the delivery and release of the drug. At the same time, because the number and position of the drug on the antibody are controllable, the ADC can achieve optimal structure and function, improving the selectivity and effectiveness of the drug. In addition, since native lysine is an amino acid naturally found in proteins, using it as a connection point will not cause rejection by the immune system, nor will it affect the structure and activity of the antibody itself.
BOC Sciences leverages years of bioconjugation experience to provide systematic, controllable, and reliable lysine conjugation strategy development services for biopharmaceutical research teams. Based on antibody structure, target DAR, payload characteristics, and production feasibility, we customize lysine conjugation schemes with different chemical reaction pathways to ensure higher reaction efficiency, more uniform product distribution, and more stable ADC performance.
Lysine conjugation is the most mature and widely used chemical route in ADC development, but it comes with inherent challenges such as multiple reaction sites, high heterogeneity, and sensitivity to antibody structure. BOC Sciences helps clients achieve the optimal balance between efficiency, uniformity, stability, and activity through systematic predictive models, highly controllable reaction systems, and comprehensive QC platforms.
Due to the widespread distribution and high reactivity of lysines, traditional NHS systems often produce multiple DARs and positional isomers, affecting uniformity and characterization.
Our solutions:
High drug loading can increase hydrophobicity, aggregation risk, and rapid in vivo clearance.
Our solutions:
Conjugation at CDRs or nearby critical regions can directly reduce affinity and neutralizing activity, a risk that must be avoided in ADC design.
Our solutions:
Lysine chemistry is influenced by pH, temperature, solvent environment, and molar ratio. Lack of control can cause DAR fluctuations and structural damage.
Our solutions:
Skilled chemists and biologists provide site accessibility prediction, route optimization, and risk assessment, reducing iterative costs. Strategies are tailored to different antibodies and payloads, improving efficiency and yield.
Platforms validated across numerous ADC projects, with optimized reaction pathways, buffers, and kinetic parameters, ensuring high yields, stable processes, and excellent batch consistency.
Lysine conjugation chemistry is widely applicable to antibodies, Fc fragments, enzymes, peptides, and fusion proteins. Optimized conditions reduce structural damage risk and enable complex molecule feasibility.
Kinetic control, site accessibility analysis, and precise molar ratio adjustment produce concentrated DAR distributions with reduced heterogeneity. Multidimensional QC ensures integrity, stability, and functionality.
Services cover activated payloads, linker synthesis, conjugation process development, and analytical method establishment, allowing clients to complete R&D on a single platform.
Complete QC data on DAR, purity, aggregation, modification sites, stability, and full analytical spectra. Reports meet regulatory submission requirements and support scale-up.
Mature SOPs, automated workflows, and large-scale platforms enable rapid project response. Flexible timelines allow efficient experimental design to sample delivery, accelerating early screening and project progression.
Efficient reaction systems, internal payload/linker synthesis, and scalable production provide significant cost advantages, suitable for exploratory research, candidate screening, and pilot-scale development.

Communicate with clients regarding antibody type, payload structure, linker characteristics, and target DAR to evaluate feasibility and draft an initial technical route. Provide scientific recommendations on antibody accessibility, structural sensitivity, and payload stability.
Design experimental plans based on multiple pathways including NHS, NCS, reductive amination, and Click-like chemistry. Systematically screen pH, molar ratio, temperature, and buffer systems to determine optimal reaction conditions.
Conduct small-scale lysine conjugation experiments and use HIC, SEC, LC-MS, CEX, and other analyses to confirm DAR, aggregation, modification sites, and antibody activity.
Adjust reaction kinetics, NHS/NCS amounts, time, and solvent systems based on small-scale results to achieve concentrated and stable DAR distributions. Optimization strategies include hydrophobicity management and structural protection.
Scale up the process while maintaining reproducibility of small-scale conditions to ensure consistent DAR, purity, and structural integrity. Provide GMP-like batch records and process parameters.

Perform final purification using HIC/SEC and complete full QC analysis (DAR, purity, aggregation, modification, activity). Deliver comprehensive analytical reports, raw spectra, and technical support, meeting standards for IND-ready material.
Lysine conjugation is a post-translational modification process involving the covalent attachment of the amino acid lysine to another molecule (usually a protein, peptide, or small molecule). Lysine conjugation can occur through a variety of mechanisms, including acetylation, ubiquitination, sumoylation, and neddylation.
Cysteine conjugation involves linking the molecule to the thiol group (-SH) of the cysteine residue in the antibody. Lysine conjugation involves attaching a molecule to the epsilon amino group (-NH2) of a lysine residue in an antibody. The main difference between these two methods is the reactivity of the amino acid residues involved. Due to the presence of thiol groups, cysteine is highly reactive and highly specific. Cysteine therefore becomes a preferred target for bioconjugation when high reactivity is required. In contrast, lysine conjugation is less specific because the amino group of lysine can react with a wider range of functional groups. This lack of specificity can be either an advantage or a disadvantage, depending on the desired application.
Drugs can be conjugated to antibodies through lysine residues using amine-reactive linkers like NHS esters or maleimides. The process typically involves activating the drug with a suitable linker, reacting it with antibody lysines under controlled conditions, and purifying the ADC. Lysine conjugation allows for multiple attachment sites, giving a mixture of DARs while maintaining antibody specificity.
Lysine conjugation may modify residues near critical regions like CDRs, potentially affecting antibody-antigen interactions. At BOC Sciences, we perform site accessibility analyses and small-scale screening to identify high-risk lysines. By selectively avoiding these residues, we ensure that antibody affinity and functional activity are preserved throughout the conjugation process.
DAR control is achieved by carefully optimizing reagent ratios, pH, temperature, and reaction duration. HIC and LC-MS analyses allow accurate monitoring of DAR distribution. Our approach ensures a concentrated, reproducible DAR range—typically 2–4—reducing heterogeneity and providing a stable, predictable conjugate suitable for preclinical and IND development.
BOC Sciences supports multiple lysine conjugation strategies, including NHS Ester, Isothiocyanate, Reductive Amination, and Click-like reactions. These approaches are compatible with traditional cytotoxins like MMAE/MMAF, DNA-damaging drugs, and peptide-based payloads. Linkers such as PABC, PEG, TCO/Tetrazine, as well as cleavable or non-cleavable types, can all be applied depending on project requirements.
Yes. Lysine conjugation is broadly compatible with IgGs, antibody fragments, fusion proteins, enzymes, and peptides. We optimize reaction conditions for each biomolecule type to preserve structural integrity, minimize aggregation, and maintain functional activity. This flexibility allows clients to explore ADCs based on diverse scaffolds without compromising product quality.
Yes. BOC Sciences offers seamless scale-up from mg-scale screening to g-scale production. Optimized reaction parameters and standardized workflows ensure consistent DAR, purity, aggregation profile, and antibody activity across scales. This capability supports both early-stage research and IND-enabling studies, providing reliable conjugates for preclinical development and regulatory submission.



Background
A North American biopharmaceutical company encountered challenges with the uncontrollable lysine conjugation process while advancing its first ADC program for solid tumor treatment. Due to the large number and wide distribution of lysine residues on the antibody surface, the universal NHS-ester conjugation system they used showed significant DAR fluctuation, low conjugation efficiency, and partial loss of antibody activity. Initial in vitro analysis revealed that the inter-batch DAR dispersion was too high and the aggregate proportion was unstable, severely affecting further pharmacodynamic and safety studies of the candidate ADC. To address this critical technical bottleneck, the company partnered with BOC Sciences with the goal of establishing a highly reproducible lysine conjugation system featuring stable DAR distribution and scalability for large-scale manufacturing.
How BOC Sciences Helped
The BOC Sciences team first performed a systematic analysis of the antibody structure, lysine exposure level, and the reactivity characteristics of the payload molecule to identify the NHS-ester chemistry most compatible with the antibody. Then, by fine-tuning key parameters such as pH, buffer composition, and feeding strategy, the team designed a lysine conjugation process tailored to the antibody, enabling the reaction to proceed within a narrow and controlled window. Meanwhile, BOC Sciences adjusted the overall hydrophilicity and ionic strength of the reaction system to reduce nonspecific modifications and enhance antibody conformational stability. Through DAR distribution control strategies, the originally highly random conjugation reaction gradually shifted toward predictability and controllability.
Implementation
Results
Taking Kadcyla as an example, the DM1 toxin molecule is connected to the lysine residue in the trastuzumab sequence through the SMCC linker. After forming a drug-linker, it will cause a mass difference of +956.36 Da (MCC-DM1). When analyzing peptide map data through mass spectrometry data processing software, set MCC-DM1 as a variable modification, perform automatic search by the software, and perform manual data confirmation through other differences caused by coupling reactions.
a. Chromatographic behavior: After coupling hydrophobic drugs, in RPLC chromatography mode, the retention time of the coupled peptides increased compared with the uncoupled peptides. At the same time, there are two types of MCC-DM1 connected through maleimide. The three-dimensional configuration causes the coupled peptides to emit peaks in pairs.
b. Coupling DM1 will prevent Trypsin enzymatic cleavage of the corresponding Lys site, so the coupled peptide will contain a missed cleavage site (except for the subsequent C-terminal Pro).
c. DM1 will produce characteristic fragments during the secondary dissociation process. The secondary mass spectrum of the coupled peptide will contain DM1 characteristic fragment ions.
Based on the software search and combined with the above manual confirmation strategy, the coupled peptides can be accurately identified. In addition, due to differences in ADC coupling methods and the diversity of linkers and optional toxic small molecules, different ADC drug molecules will have their own unique situations, and a suitable coupling peptide screening program needs to be developed based on the actual ADC molecular structure.
References
Browse BOC Sciences' publications to explore articles from research teams worldwide, showcasing the scientific contributions of our products and services in cutting-edge drug development.

"BOC Sciences delivered exactly what we needed for our lysine-based ADC prototype — fast turnaround, clean conjugation, and reliable DAR control. Their expertise saved us weeks of optimization."
— Dr. Emily Carter, Senior Antibody Engineer (United States)
"The team at BOC Sciences made our lysine conjugation project effortless. From reaction setup to analytical support, everything was smooth, clear, and on schedule. Truly a dependable partner."
— Mr. Daniel Weber, Bioconjugation Development Scientist (Germany)
"We chose BOC Sciences for their reputation in ADC chemistry, and they exceeded expectations. The lysine conjugates were highly consistent and ready for screening without any rework."
— Ms. Laura Jennings, Preclinical Research Lead (United Kingdom)
"Outstanding service. BOC Sciences provided stable, high-quality lysine conjugates that greatly accelerated our payload–antibody assessment. Professional, responsive, and scientifically solid."
— Dr. Marco Rossi, Principal Scientist, Chemical Biology (Italy)
References
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










