As a leading service supplier in antibody-drug conjugates (ADCs) development, BOC Sciences provides flexible ADC linkers product suite and bioconjugation service at competitive prices. We provide various ADC linkers development services for global customers to achieve new drug discovery milestones with comprehensive and advanced platforms.
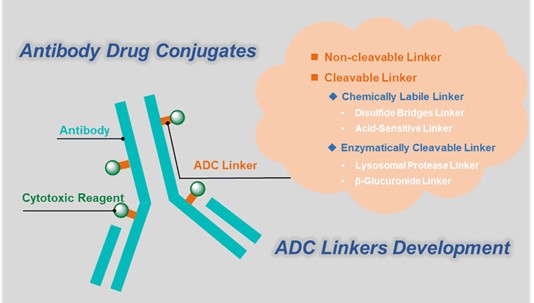
With the improvements in payloads, linkers and conjugation methods, the development of ADC has achieved significant progress during the past decade. In particular, linker design plays a crucial role in regulating the stability of ADC in systemic circulation and payload release efficiency within tumors, thereby affecting the pharmacokinetics (PK), efficacy and toxicity characteristics of ADCs. Currently, two types of linkers are used according to different mechanisms, and they are cleavable linkers and non-cleavable linkers. Key ADC linker parameters, such as conjugation chemistry, linker length, and linker steric hindrance, affect the PK and efficacy of ADC drugs. Therefore, in the design of ADC drugs, BOC Sciences provides customized services that support the correct adjustment of essential linker parameters to balance the stability and payload release efficiency, thus achieving suitable and successful ADC drugs developments.
Linkers not only serve as the bridge between cytotoxins and antibodies but also determine the timing and manner of drug release. BOC Sciences focuses on the development and customization of various types of ADC linkers, covering three main categories: chemically cleavable linkers, enzymatically cleavable linkers, and non-cleavable linkers. Combining advanced conjugation technologies with process development capabilities, we provide clients with one-stop services from structure screening, reaction optimization to scale-up production, helping to build efficient, safe, and controllable ADC products.
We offer a variety of chemically cleavable linkers sensitive to acid and hydrolysis, supporting precise drug release under acidic conditions in the tumor microenvironment or inside cells. These are commonly used to enhance the controllability and efficiency of drug release.
We develop linkers cleavable by tumor-associated enzymes such as Cathepsin B and β-glucosidase, including peptide chains like Val-Cit and Gly-Phe, ensuring specific release within target cells to improve therapeutic targeting and safety.
We provide stable non-cleavable linkers, such as SMCC, which release active drug fragments through intracellular antibody degradation, suitable for ADC constructs with extremely high stability requirements.
Different types of linkers target specific enzymatic activities or microenvironment conditions inside tumor cells to achieve targeted and efficient drug release. BOC Sciences has extensive experience in linker development and can provide various specific enzymatically cleavable linkers, chemically sensitive linkers, and reduction-sensitive linkers to meet different ADC design needs. We support the supply of mature linkers and can customize innovative linkers according to customer requirements, helping to optimize ADC efficacy and safety. With an advanced synthesis platform, rigorous quality control, and flexible process scale-up capabilities, BOC Sciences offers clients professional one-stop services from linker design, synthesis, conjugation to scale-up production, ensuring efficient advancement of ADC projects.
| Linker Type | Description | Our Development Capability |
| Cathepsin B Cleavable Linkers/Peptide Linkers | Peptide linkers designed for tumor-associated Cathepsin B enzyme, enabling specific intracellular drug release. | Custom peptide design, optimizing cleavage sites to improve conjugation efficiency and in vivo stability. |
| Phosphatase Cleavable Linkers | Cleaved by phosphatases, suitable for tumor environments with phosphatase activity for precise drug release. | Precise synthesis of phosphate ester linkers with support for structure optimization and functional validation. |
| Sulfatase Cleavable Linkers | Cleaved by sulfatase enzymes in tumor microenvironment to achieve targeted drug release. | Provides diversified sulfate ester linker synthesis ensuring selectivity and stability. |
| β-Galactosidase Cleavable Linkers | Cleaved by β-galactosidase, ideal for triggering drug release in tumors expressing this enzyme. | Supports efficient synthesis and modification of glycosidic linkers to optimize enzyme sensitivity. |
| β-Glucuronidases Cleavable Linkers | Cleaved by β-glucuronidase, allowing specific drug release inside tumor cells. | Develops various glucuronide linkers balancing in vivo stability and efficient release. |
| Acid Cleavable Linkers/Hydrazone Linkers | Cleaves in acidic environments such as lysosomes, used for pH-triggered drug release. | Offers mature acid-sensitive linker synthesis and modification technologies ensuring stability and responsiveness. |
| Disulfide Linkers | Reductively cleaved in reducing environments (e.g., high glutathione levels), enabling drug release. | Precisely controls disulfide bond structure and reduction sensitivity to ensure conjugate stability and targeted release. |
With the continuous advancement of ADC research and development, single-dimensional linker design can no longer meet the clinical demands for high efficacy and low toxicity. Systematic and integrated linker optimization solutions have become especially important. BOC Sciences is dedicated to providing clients with comprehensive, customized linker design services, covering everything from linker site selection, chemical structure modulation to dosage regimen integration, comprehensively enhancing the pharmacokinetic properties and therapeutic effects of ADCs, helping clients achieve the development of a new generation of high-performance ADC drugs.
ADC undergoes biotransformation through antibody metabolism, linker cleavage, and payload metabolism. Ideal ADCs stay stable in circulation until reaching target cells. BOC Sciences offers solutions including conjugation site selection and linker modification—adjusting site, length, and steric hindrance—to improve ADC stability and efficacy.
Binding sites can also become affecting factors in payload release kinetics. Generally, binding sites directly affect release kinetics independent of the monoclonal antibody. Moreover, the introduction of sterically hindered chemical modification at the cleavage site can regulate ADC stability, thus leading to delay or ineffective release of the payload.
In order to obtain the most suitable ADC drug, linker design and integration are necessary. Linker integrated design includes multiple parameters, such as conjugation site, linker length, linker chemistry, cleavable/non-cleavable linkage, and steric hindrance of the proximal linkage. BOC Sciences provides optimized linker design schemes to balance ADC stability and payload release kinetics so that the release of the payload within tumor cells reaches a higher therapeutic threshold.
Higher ADC doses do not always improve drug efficacy. In fact, once a critical threshold is reached, an increase in dosage may lead to potential toxicity. Therefore, it is necessary to integrate linker and dose designs thus discovering the minimum effective dosage. When single-dose and multiple-dose regimens reveal similar effects, BOC Sciences is capable of helping our customers to choose the most suitable dosage that achieves the best curative effect and minimal toxicity.
The breakage of linkers directly controls cytotoxic molecules release. In order to reduce the off-target toxicity of ADC, it is necessary to design selective break sites. BOC Sciences can provide global customers with support services at the following break sites: histone enzymolysis sites, acidolysis sites, GSH cleavage sites, divalent iron cleavage sites, novel enzymatic sites, photosensitive sites, and non-cleavable linkers.
Currently, the connection between linker and antibody confront two major obstacles, reverse Michael addition reaction of the maleimide linker and uneven DAR value. In order to solve the first problem, BOC Sciences adopts various substituents to achieve upregulated stability while maintaining the high reactivity and specificity of maleimide linkers.
The connection between linker and payload is minimal, and the most common ones are amide bonds and ester bonds. As payload structures become more diversified, the restricted connection mode limits their connections with linkers. BOC Sciences develops linkers that adapt to diversified payloads, so as payloads that suit existing linkers.
Supports laboratory-scale production from gram to milligram levels, flexibly meeting needs at different R&D stages to ensure smooth project progression.
Provides high-quality linkers and related products at competitive prices, balancing performance and economy.
Covers bioanalytical testing related to ADCs, ensuring precise evaluation of product quality and performance.
Applies cutting-edge technologies and innovative methods to improve the efficiency and stability of linker design and synthesis.
Leverages GMP-certified processes for large-scale synthesis of cytotoxins and linkers, complying with pharmaceutical manufacturing standards.
Implements strict conjugation process management in a GMP environment, coupled with comprehensive QA/QC to ensure ADC quality consistency.
Possesses mature linker design and cytotoxin synthesis technologies to facilitate efficient ADC development.
Has multi-project experience in ADC conjugation and process scale-up, ensuring smooth transition from R&D to industrialization.


By rational linker structure design, optimizing conjugation sites, and adjusting chemical properties, stability in circulation is enhanced to prevent premature drug release and improve overall ADC efficacy.
Customized services are supported. We design innovative linker structures based on customer needs, optimize release mechanisms, and maximize targeting and efficacy.
By considering drug properties, target environment, and therapeutic goals, we evaluate linker stability and release mechanisms, providing personalized professional advice to ensure the best match.
The cycle depends on project complexity, usually ranging from several weeks to months, including design, synthesis, validation, and optimization to guarantee product quality and R&D efficiency.
Large-scale production is supported. With mature process platforms and strict quality control, we ensure efficient and stable scale-up of linker synthesis to meet commercialization needs.
Linkers determine the speed and location of drug release, impacting ADC targeting and toxic payload release, directly affecting therapeutic outcomes and patient safety.
Structural optimization is provided. Combining synthetic processes and bioactivity testing, we adjust linker design to improve stability, specificity, and efficacy.
Clients can contact us to submit requirements. Our technical team offers consultation, scheme design, and customized development, supporting the full process from R&D to production.
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










