Chemically cleavable linkers are key structural units in the design of Antibody-Drug Conjugates (ADCs). They are responsible for precisely connecting cytotoxic payloads to antibodies via controllable chemical bonds, enabling drug release under specific chemical conditions. With predictable chemical cleavage mechanisms, these linkers play a central role in regulating drug release rates, optimizing plasma stability, and ensuring precise delivery within tumor cells. Leveraging years of experience in antibody conjugation and linker development, BOC Sciences offers a one-stop customized service—from linker design, synthesis, modification, and optimization to quality analysis—meeting diverse ADC development needs across different targets, pharmacodynamics, and release mechanisms.
Chemically cleavable linkers are linker structures that break in response to chemical reactions. Typically, these linkers contain chemical bonds sensitive to specific chemical stimuli such as changes in pH, reducing environments, oxidative conditions, or particular reagents. Upon reaching the target site, the linker undergoes hydrolysis, reduction, oxidation, or rearrangement triggered by the local chemical environment, releasing the active drug molecules. Unlike enzyme-cleavable linkers, chemically cleavable linkers rely on differences in the chemical microenvironment of tumors or lesions (e.g., pH, redox status, reactive oxygen species levels) to achieve site-specific drug release.
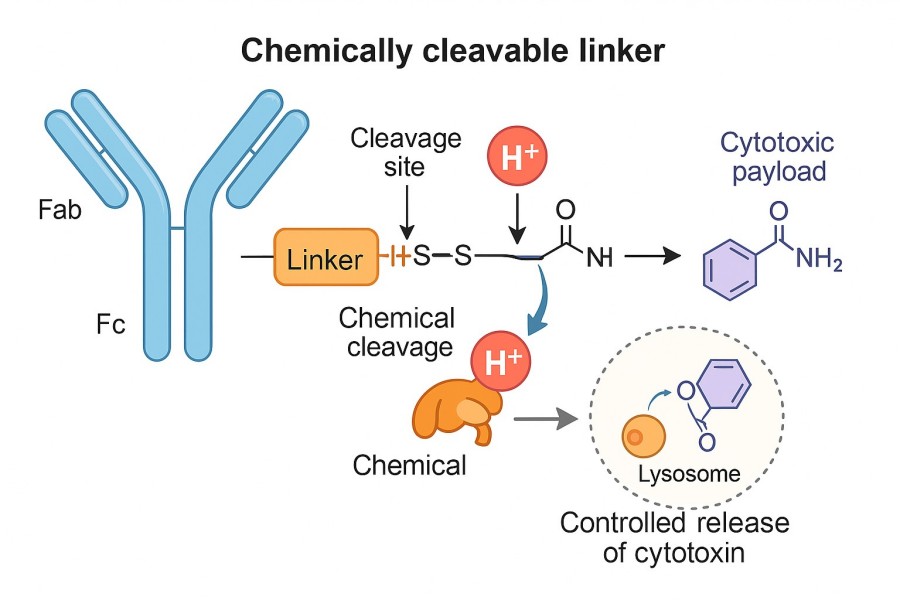 Fig. 1. Chemical-sensitive linker in ADC (BOC Sciences Authorized).
Fig. 1. Chemical-sensitive linker in ADC (BOC Sciences Authorized).
Chemically labile linkers are widely used in ADC clinical pipelines, with acid-sensitive linkers (such as hydrazones and silyl ethers) and disulfide linkers leading the field. Hydrazones are easily synthesized and exhibit a plasma half-life of 183 hours at pH 7 or 4.4 hours at pH 5, indicating selective cleavage under acidic conditions like those in lysosomes. The first-generation ADC gemtuzumab ozogamicin (Mylotarg) incorporates a hydrazone linker critical for drug potency. However, acidic conditions also exist in various bodily fluids, raising concerns of nonspecific drug release. Disulfide linkers exploit the reducing environment inside cells to release drugs in lysosomes following internalization and antibody degradation. Most disulfide-linked drugs are initially freed intact through proteolytic cleavage of the antibody and subsequently released as active metabolites via disulfide exchange or reduction by agents such as glutathione. The methylated drug metabolites can then diffuse through lipid membranes to reach their site of action.
BOC Sciences possesses a rich chemical linker structure database and a mature synthesis platform, providing a wide range of classical and novel chemically cleavable linkers for ADC development. These cover various mechanisms including acid-sensitive, reduction-sensitive, and oxidative stress-sensitive types, matching different pharmacodynamic requirements and drug chemical properties. Common linkers we offer include:
Acid-cleavable linkers (pH-sensitive linkers) utilize the acidic intracellular tumor environment (pH 4.5–6.5) contrasted with the neutral blood environment to rapidly hydrolyze and release drugs under acidic conditions. BOC Sciences selects appropriate chemical structures such as hydrazone, acyl hydrazone, or acetal linkages based on the drug's structure, optimizing linker length and hydrophobicity to ensure stability in circulation and efficient release at the target site.
Reduction-sensitive linkers depend on the high concentration of glutathione (GSH) within tumor cells to trigger disulfide bond cleavage and drug release. BOC Sciences specializes in customizing disulfide bond structures and protective groups tailored to drug properties, employing in vitro and in vivo reductive environment simulation platforms to test stability and cleavage rates, guaranteeing precise response to cytosolic reducing conditions.
Oxidation-sensitive linkers leverage elevated reactive oxygen species (ROS) levels in tumor and inflammatory sites, utilizing boronate esters, thioethers, or selenoethers that cleave under oxidative conditions to release drugs. BOC Sciences screens ROS-sensitive chemical structures compatible with the drug and adjusts their activation thresholds to balance site-specific release and blood stability.
β-Elimination linkers release drugs via β-elimination reactions triggered under certain chemical conditions, with reaction rates tunable through chemical modifications. BOC Sciences uses high-throughput synthesis platforms to customize diverse β-elimination structures and combines kinetic testing to optimize stability and release performance, meeting precise control requirements on release timing and dosage.
With advanced chemical synthesis platforms and comprehensive analytical systems, BOC Sciences provides full-process chemically cleavable linker services including design, structural optimization, custom synthesis, and conjugation process development. We understand that linkers influence not only ADC drug release mechanisms and plasma stability but also directly impact clinical efficacy and safety. Therefore, we strictly control quality and reproducibility at every R&D stage to ensure clients receive linker products with stable performance, strong controllability, and tailored to project needs.
BOC Sciences has years of experience in linker design, synthesis, and optimization, successfully supporting multiple ADC projects advancing to clinical stages, including acid-sensitive, reduction-sensitive, and hybrid mechanisms, ensuring mature and feasible solutions.
We tailor linker structures based on client drug structure, target characteristics, and release requirements—including length, spatial configuration, hydrophobicity/hydrophilicity, and reactive groups—to achieve optimal efficacy and stability balance.
Providing full-process services from linker screening, custom synthesis, conjugation process development to quality control, covering milligram-scale research to kilogram-scale scale-up production, shortening ADC development timelines and reducing project risks.
Manufacturing processes comply with cGMP and ISO quality systems, offering complete COA, SDS, and R&D documentation to ensure chemically cleavable linkers are directly applicable for preclinical and clinical research.
Optimizing synthetic routes and reaction conditions to improve efficiency, shorten project turnaround, and reduce production costs, helping clients gain a time advantage in the competitive ADC market.
Supported by an international team and advanced analytical platforms, we serve global clients' ADC linker customization needs and provide comprehensive technical support from project initiation to regulatory submission, ensuring smooth R&D progression.

Before project initiation, the BOC Sciences team engages in deep discussions with clients to thoroughly understand drug structure, target environment, expected release mechanisms, and project timelines. Leveraging professional experience and technical reserves, we assess project feasibility and technical challenges to lay a solid foundation for design.
Based on project requirements, we design multiple linker solutions compatible with chemical cleavage mechanisms. Combining molecular modeling and literature data, we screen for optimal structures. Clients participate in solution reviews to confirm design details, ensuring alignment with drug conjugation and efficacy requirements.
Entering laboratory phase, we conduct small-scale synthesis with strict reaction monitoring to guarantee linker purity and structural accuracy. Using NMR, LC-MS, and other analytical methods, we verify molecular structures and ensure product compliance with design criteria.
Based on small-scale results, we develop pilot and scale-up production processes. Concurrently, we optimize conjugation conditions with antibodies, adjusting reaction parameters to achieve ideal drug loading and conjugate stability.
Conduct in vitro stability assessments on synthesized linkers and conjugates, including plasma stability and storage stability tests. Simulate tumor microenvironment to measure chemical cleavage rates, ensuring efficient and controllable drug release mechanisms.

Complete comprehensive quality control throughout the process, providing detailed analytical reports and Certificates of Analysis (COA). Project deliverables are ready for direct application in preclinical research or further development. BOC Sciences ensures clients receive high-quality products on schedule to meet R&D objectives.
The primary function of a chemically cleavable linker in ADCs is to securely attach the cytotoxic drug to the antibody while enabling controlled drug release under specific chemical conditions. These linkers remain stable in systemic circulation to prevent premature drug release but are cleaved inside target cells triggered by stimuli such as acidic environments, reductants, or reactive oxygen species. This precise drug release enhances therapeutic efficacy and reduces systemic toxicity.
Chemically cleavable linkers do not depend on the activity of specific enzymes in vivo, avoiding uncertainties caused by variable enzyme expression. Their cleavage conditions are more controllable and predictable. For example, acid-sensitive linkers utilize the low pH environment within tumor cells to trigger drug release, with stable and easily adjustable reaction mechanisms. Compared to enzyme-cleavable linkers, chemically cleavable linkers offer more consistent performance across different patients and tissues, making them especially suitable for ADC designs requiring precise control of drug release rate and location, thereby improving therapeutic outcomes and lowering side effect risks.
Common chemically cleavable linkers include acid-sensitive, reduction-sensitive, oxidative stress-sensitive, and metal ion-sensitive types. Acid-sensitive linkers leverage the low pH within tumor cells for drug release; reduction-sensitive linkers rely on high intracellular glutathione concentrations to reduce and cleave disulfide bonds; oxidative stress-sensitive linkers undergo cleavage induced by reactive oxygen species; metal ion-sensitive linkers depend on specific metal ions to catalyze cleavage.
Chemically cleavable linkers are widely applied in ADC development as controlled-release bridges between drugs and antibodies, enhancing targeting and safety. Beyond ADCs, they are also used in peptide-drug conjugates, nucleic acid drug delivery systems (such as siRNA and mRNA), nanomedicine carriers, and prodrug designs, providing critical technological support for precision medicine and cancer therapy.
A pH cleavable linker is a type of chemically cleavable linker specifically designed to break in acidic environments. It exploits the lower pH (typically 4.5 to 6.0) inside lysosomes or endosomes of target cells as the trigger for precise drug release. Common pH cleavable linker structures include hydrazone and acetal linkages. This mechanism ensures the linker remains stable in blood circulation but efficiently releases the drug within target cells, improving the safety and efficacy of ADCs.
Absolutely. BOC Sciences has extensive experience in chemical modifications and can flexibly design and introduce various functional groups such as maleimide, alkyne, azide, amine, etc., according to the client's drug structure and antibody characteristics. These groups not only ensure efficient conjugation but also improve the stability and drug release performance of the conjugate. We are committed to providing highly matched, high-performance linkers to ensure the best outcome for your ADC project.
Yes. BOC Sciences offers comprehensive one-stop services covering linker design, custom synthesis, conjugation process development, and final ADC preparation. Our team includes experts in organic synthesis, analytical testing, and bioconjugation, capable of tailoring complete solutions based on client needs. This integrated service significantly enhances R&D efficiency, ensures consistent conjugation quality, and provides strong support for preclinical development and commercial production of ADCs.
We conduct rigorous in vitro stability testing to evaluate the chemical stability of linkers under plasma and physiological conditions, ensuring they remain intact during circulation. Meanwhile, we validate controlled cleavage ability under simulated tumor microenvironment conditions (e.g., low pH, high glutathione levels). By optimizing molecular design—adjusting steric hindrance and hydrophilicity/hydrophobicity of linker structures—we minimize off-target release risk, safeguarding the safe and effective delivery of drugs to target cells.
Background
A biopharmaceutical company was developing a novel ADC with a core requirement to design a chemically cleavable linker capable of specifically releasing cytotoxins inside tumor cells. Given the candidate ADC's cytotoxic payload demanded extremely high conjugation stability and controlled release, conventional enzyme-cleavable linkers could not meet the dual needs for precise release and plasma stability. The project urgently required innovative custom development of a hydrazone linker to ensure efficient targeting and safety.
How BOC Sciences Helped
Leveraging extensive ADC linker development experience, BOC Sciences collaborated closely with the client to deeply analyze the drug's structure and tumor microenvironment characteristics. Multiple acid-sensitive chemically cleavable linker schemes centered on hydrazone linkers were designed and screened. Molecular modeling predicted hydrazone linker cleavage kinetics under various pH conditions, optimizing structures to balance plasma stability and tumor-targeted release efficiency. Meanwhile, hydrazone linkers featuring multifunctional reactive groups were custom synthesized to ensure efficient conjugation and adaptability to downstream processing.
Implementation Process
Key Results
With exceptional quality and stable performance, BOC Sciences' ADC linker products and custom services have gained broad recognition from researchers worldwide and have been cited in multiple studies published in top international journals. These publications not only highlight BOC Sciences' technological expertise in the linker field but also underscore its significant role in driving innovative ADC drug development and fostering global academic collaboration.

"The BOC Sciences team demonstrated exceptional expertise in customizing chemically cleavable linkers for our ADC projects. Their precise control over linker stability and drug-to-linker ratio ensured consistent performance, which is critical for our preclinical studies."
— Dr. Emily Carter, Senior Scientist, Drug Discovery (USA)
"BOC Sciences provided comprehensive support in synthesizing acid-sensitive cleavable linkers tailored to our molecules. Their rigorous quality controls and transparent communication made the collaboration highly efficient and reliable."
— Dr. Thomas Müller, Principal Chemist (Germany)
"Our collaboration with BOC Sciences on reductive-sensitive linker development was seamless. Their deep technical knowledge and flexible approach helped optimize linker design, accelerating our ADC pipeline progress."
— Dr. Laura Johnson, Research Fellow (UK)
"BOC Sciences delivered high-quality chemically cleavable linkers with excellent stability and release profiles. Their professional service and prompt technical feedback greatly benefited our ADC candidate optimization."
— Dr. François Dubois, Medicinal Chemistry Specialist (France)
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










