Site-specific conjugation refers to the strategy of precisely attaching functional molecules to defined sites on antibody molecules through chemical, enzymatic, or protein engineering approaches, resulting in structurally well-defined and highly homogeneous antibody conjugates. Compared with traditional random conjugation, this approach enables precise control over conjugation sites and drug-to-antibody ratio (DAR), significantly enhancing molecular stability and development potential. BOC Sciences offers comprehensive site-specific conjugation services, covering multiple technology platforms including engineered cysteine conjugation, enzymatic conjugation, non-natural amino acid incorporation, and glycan-directed conjugation. Our experienced team of scientists can handle a wide variety of antibody types, including full-length antibodies, antibody fragments, and engineered antibodies, while providing end-to-end support in small-scale optimization, process development, and analytical characterization, delivering highly homogeneous and reproducible antibody conjugates to accelerate antibody-drug conjugate (ADC) and targeted delivery projects.
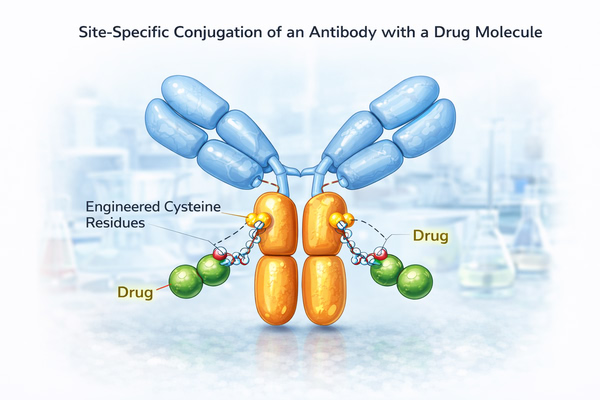
Site-specific conjugation is an antibody conjugation strategy that covalently links functional molecules only at pre-defined sites on the antibody through chemical, enzymatic, or protein engineering methods. Unlike traditional lysine- or cysteine-based random conjugation, site-specific conjugation achieves precise control over conjugation location and number at the molecular level, yielding structurally defined and highly homogeneous antibody conjugates. Under this strategy, each antibody molecule typically possesses consistent conjugation sites and predictable DAR, substantially reducing molecular heterogeneity. This enhances product stability, controllable in vivo behavior, and batch-to-batch consistency, making it a key technology pathway in the development of ADCs, targeted delivery systems, and advanced diagnostic conjugates.
BOC Sciences provides site-specific conjugation services across multiple technology platforms, enabling flexible design of highly homogeneous, reproducible, and scalable antibody conjugates tailored to different antibody structures, payload types, and application needs. Leveraging our mature antibody engineering, chemical conjugation, and analytical characterization platforms, we support custom conjugation process development from early feasibility studies to preclinical research, ensuring that conjugation sites, DAR, and molecular stability meet overall project objectives.
BOC Sciences offers multiple site-specific conjugation approaches, including engineered cysteine, enzymatic conjugation, non-natural amino acids, glycan-directed modification, and disulfide re-bridging, allowing flexible selection of optimal strategies based on antibody structure and payload characteristics.
Our systematic capabilities span feasibility assessment, small-scale verification, and process parameter optimization, comprehensively refining DAR distribution, conjugation efficiency, stability, and aggregation risk to ensure reproducible and scalable conjugation outcomes.
Utilizing multidimensional analytical platforms, we assess DAR, purity, homogeneity, structural integrity, and stability of conjugates, providing reliable data to meet R&D and regulatory quality requirements.
Fully equipped biochemical and protein conjugation labs support research and preclinical projects, with strict data management and quality control protocols to ensure stable and consistent results.
BOC Sciences provides project-based coordination and technical communication support, adjusting strategies according to research stage and timelines, rapidly responding to changing requirements to ensure efficient project progression.
Our team combines antibody engineering with high-selectivity chemical conjugation expertise, enabling unified development of sequence design, site construction, and conjugation processes, shortening project timelines and reducing technical risk.

Engage with clients to discuss antibody types, conjugation goals, payload properties, and application scenarios, evaluating antibody structure and site-specific strategies to determine project feasibility and recommend optimal approaches.
Based on assessment outcomes, select suitable site-specific conjugation technology, such as engineered cysteine, enzymatic conjugation, or glycan-directed modification, and develop preliminary process design and quality target parameters.
Conduct conjugation experiments at small scale, systematically optimizing reaction conditions, feed ratios, and reaction times, focusing on conjugation efficiency, DAR distribution, and molecular stability.
Apply tailored purification strategies to obtain target conjugates, performing comprehensive characterization of purity, homogeneity, structural integrity, and stability using multiple analytical methods.
Provide clients with complete experimental data, analytical results, and technical summaries, ensuring clear traceability of conjugation sites, DAR, and quality metrics to support further research or process scale-up decisions.

Offer additional process optimization, strategy adjustments, or technical consultation based on project progress, assisting clients in advancing to higher research stages or subsequent applications.
Site-specific conjugation reduces molecular heterogeneity caused by random modification of lysine or cysteine residues. By controlling both conjugation sites and DAR, it minimizes variability, preserves antibody binding and Fc functions, and improves safety and efficacy profiles, which is particularly important for ADC development and regulated clinical programs.
BOC Sciences provides multiple site-specific conjugation platforms, including engineered cysteine conjugation, enzymatic conjugation, non-natural amino acid incorporation, glycan-based conjugation, disulfide re-bridging, and pClick™ technology. This multi-platform capability allows us to select or design the optimal strategy based on antibody structure and payload properties.
Yes. Site-specific conjugation enables precise control of DAR, typically achieving defined values such as DAR 2 or DAR 4. This control is critical for balancing efficacy and toxicity in ADCs, improving pharmacokinetics, and ensuring reproducible manufacturing outcomes across development batches.
Site-specific conjugation supports a wide range of payloads, including highly potent small-molecule drugs, fluorescent dyes, imaging probes, radionuclide chelators, oligonucleotides, and polymers. Payload compatibility depends on the selected conjugation chemistry, which can be optimized to preserve both antibody integrity and payload activity.
BOC Sciences offers customized site-specific conjugation solutions based on antibody format, payload chemistry, and application goals. Our support includes feasibility assessment, strategy selection, process optimization, analytical characterization, and technical consultation, ensuring each conjugation approach aligns with the client’s development objectives.
Background
A European biotechnology company was developing a HER2-targeting IgG1 monoclonal ADC for preclinical studies in solid tumors. Early in the project, the ADC was constructed using a conventional lysine-based random conjugation strategy. However, multiple batch preparations showed broad DAR distribution, high molecular heterogeneity, reduced antibody binding activity, and insufficient in vitro stability. These issues significantly interfered with subsequent efficacy evaluation and safety studies, creating an urgent need for a more controllable and structurally homogeneous conjugation solution.
How BOC Sciences Helped
During the project evaluation stage, BOC Sciences conducted a systematic analysis of the antibody structure, payload characteristics, and prior conjugation data. We proposed using an engineered cysteine site-specific conjugation strategy to achieve precise control over conjugation sites and DAR. The project team collaborated closely with the client on antibody engineering feasibility, target DAR design, and downstream analytical parameters. A comprehensive technical plan covering antibody modification, small-scale conjugation, purification, and structural characterization was developed to ensure that the new strategy significantly improved molecular consistency without compromising antibody affinity.
Implementation
Results
Browse BOC Sciences' publications to explore articles from research teams worldwide, showcasing the scientific contributions of our products and services in cutting-edge drug development.

"BOC Sciences provided exceptional support in developing a site-specific ADC using engineered cysteine conjugation. Their team guided us through every step, from feasibility assessment to analytical characterization, and delivered highly uniform conjugates on schedule. Their technical expertise is impressive."
— Dr. Emily Carter, Senior Scientist (USA)
"Facing challenges with heterogeneous ADC batches, we turned to BOC Sciences for a site-specific conjugation solution. They optimized the DAR and ensured structural consistency, providing reproducible results and comprehensive data. Their professional approach and communication were outstanding."
— Mr. Thomas Müller, R&D Manager (Germany)
"We required precise conjugation of a fluorescent probe to our antibody for in vivo imaging studies. BOC Sciences executed the enzymatic site-specific conjugation flawlessly, preserving antibody activity and delivering high-quality material on time. Their expertise and responsiveness were remarkable."
— Dr. Olivia Thompson, Molecular Imaging Specialist (UK)
"BOC Sciences helped us implement site-specific conjugation for a novel ADC. The team provided clear guidance, optimized the reaction, and delivered a highly homogeneous product with reliable DAR. Their support greatly accelerated our preclinical research timeline."
— Mr. Alexander Johnson, Preclinical Development Scientist (USA)
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










