A non-cleavable linker refers to a type of linker that remains stable in vivo and is resistant to enzymatic or chemical cleavage. Unlike cleavable linkers, non-cleavable linkers maintain stability in blood circulation, ensuring that the drug is not prematurely released in non-target tissues. The core feature of non-cleavable linkers is their ability to connect small-molecule drugs (cytotoxins) to monoclonal antibodies (mAbs) through stable covalent bonds, thereby optimizing the pharmacokinetic properties of antibody-drug conjugates (ADCs). Non-cleavable linkers are particularly suitable for ADC designs that require high target specificity and aim to minimize off-target toxicity. Because their release mechanism relies on antibody degradation within target cells, non-cleavable linkers are especially effective in enhancing ADC safety and improving the therapeutic index. BOC Sciences offers a variety of high-quality non-cleavable linker products, specifically designed for ADC research and production. Our offerings include commonly used thioether-based, maleimide-based, and other innovative linker chemistries, providing flexible solutions to meet diverse payload types, antibody structures, and target cell environments.
Comprehensive one-stop antibody-drug conjugate service platform
Large Stock
More than 1000+ high-purity products in inventory

Global Delivery
Warehouses in multiple cities to ensure fast delivery

mg to kg
Qualified facilities & equipment of cGMP laboratory

24/7 Technical Support
Strict process parameter control to ensure product quality

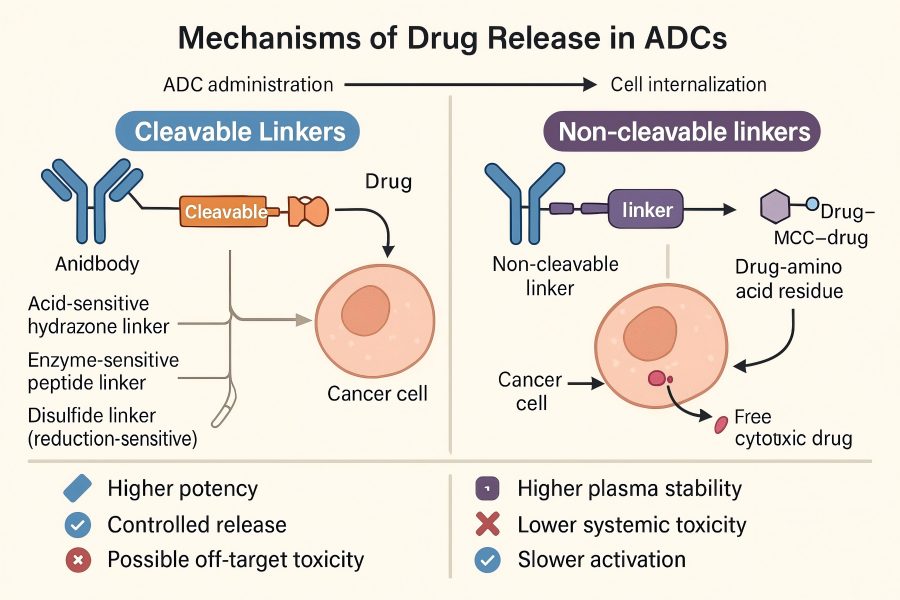
ADCs is a novel generation of antibody-targeted therapeutic drug, mainly applied for tumors and cancer treatment. Structurally, ADCs are composed of 3 components, the payload, antibody and linker. Mechanistically, ADCs act by binding to the target antigen on the cell surface, internalization via antigen-mediated endocytosis trafficking into the lysosome, and payload release through the proteolytic degradation of the antibody moiety/or cleavage of the linker. The rationale for developing ADCs is that linking a cytotoxic agent to a tumor-targeting antibody will enable selective targeting to cancer cells, leading to their eradication while sparing cells in normal tissues. The most essential role of ADCs is the release of cytotoxic molecules in the cytoplasm, which is achieved by destroying the chemical linker between the antibody and the cytotoxic molecule. Thus, choosing a suitable linker is highly critical to ADCs. The ideal ADCs linker must satisfy the following conditions: it must be stable enough in the peripheral blood circulation to avoid the toxicity caused by the release of cytotoxic molecules. Thus, it can effectively release small cytotoxins after ADCs internalization. The key to ADCs therapeutic effect is to select a suitable linker and couple with the appropriate number of cytotoxins at the reaction site. The linkers can be divided into cleavable linkers and non-cleavable linkers based on their dissociation properties.
Non-cleavable linkers stabilized in the blood circulation system and tumor cells, such as thioethers linkers and amides linkers. After being engulfed by tumor cells, ADC monoclonal antibodies (mAbs) are degraded in lysosomes, releasing small-molecule cytotoxic from the linker to exert antitumor effects. With this mechanism, differences between parent drugs and potential ADCs metabolites must be taken into consideration. For example, MMAE, a protein-based anti-mitotic drug, is most potent in its native form and is therefore poorly suited for derivatization with non-cleavable linkers. Conversely, MMAF retained its potency even when linked with a simple alkyl chain in vitro and in vivo. One proposed mechanism for the decreased efficacy of non-cleavable linked ADCs is that drugs bearing charged amino acids suffer from decreased membrane permeability, limiting their ability to kill nearby cells. Several non-cleavable alkyl and polymeric linkers have been explored in ADCs development. A notable example is the MCC amine-to-sulfhydryl bifunctional cross-linker featured in T-DM, this linker is especially useful as the cyclohexane ring provides a steric hindrance that decreases the hydrolysis of the resulting thioether.

Non-cleavable linkers possess several notable characteristics in ADC design. These features make non-cleavable linkers an important choice in modern ADC development, especially in antitumor therapy, where they offer broad application value.
The core feature of non-cleavable linkers is their chemical stability: in plasma and the circulatory system, they are not broken down by enzymes, pH changes, or hydrolysis, ensuring that the drug is not prematurely released in non-target tissues. This contrasts sharply with cleavable linkers, which rely on pH changes, enzymatic activity, or reducing environments to trigger drug release, carrying a certain risk of off-target effects.
The key to drug release by non-cleavable linkers lies in intracellular antibody degradation. When an ADC recognizes and binds a specific antigen on the target cell surface, it enters the cell via receptor-mediated endocytosis. Within endosomes, the ADC is transported to the acidic lysosomal environment. Proteolytic enzymes in the lysosome, such as proteases and acid hydrolases, degrade the antibody protein backbone, while the non-cleavable linker itself remains covalently stable and does not break. This design ensures the drug is released only inside target cells, avoiding premature release in blood circulation or non-target tissues.
The released drug typically exists as an antibody fragment–linker–drug complex, which remains bioactive and can interact with intracellular targets (e.g., microtubules or DNA) to exert cytotoxic or inhibitory effects. Because release depends on the rate of antibody degradation, non-cleavable linkers provide controlled drug release, allowing ADCs to exert a more durable and stable therapeutic effect inside cells. Additionally, linker structures can be optimized according to the chemical properties of different payloads to enhance release efficiency after lysosomal degradation, achieving precise drug delivery.
Another major advantage of non-cleavable linkers is their exceptional stability in plasma and circulation. Traditional cleavable linkers are susceptible to hydrolytic enzymes, reducing environments, or pH changes in blood, leading to premature drug release and off-target toxicity. Non-cleavable linkers, through stable covalent bond structures, effectively resist these influences, keeping ADCs intact in the bloodstream and reducing fluctuations in free drug concentration.
This stability not only extends the plasma half-life of ADCs but also improves the therapeutic index. Clinically, this means drugs are primarily concentrated in target tissues, minimizing damage to healthy tissues. Furthermore, highly stable linkers enhance pharmacokinetic predictability, allowing developers to optimize dosing regimens and safety assessments more accurately. It is worth noting that different types of non-cleavable linkers (e.g., thioether, maleimide, aryl ether) vary in plasma stability and drug release efficiency after antibody degradation. Research teams can select the most suitable linker chemistry based on payload characteristics and therapeutic needs to achieve the optimal balance of efficacy and safety.
Non-cleavable linkers offer significant advantages in ADCs, mainly in terms of plasma stability, reduced off-target toxicity, and enhanced therapeutic index. These advantages not only optimize ADC pharmacokinetics but also provide higher safety and efficacy in clinical applications.
First, non-cleavable linkers exhibit exceptional plasma stability. Compared with traditional cleavable linkers, they are less prone to degradation by enzymes, hydrolysis, or pH changes in circulation, effectively extending the in vivo half-life of ADCs. This stability reduces the risk of premature drug release and minimizes fluctuations in free drug levels in blood, providing reliable support for long-term therapy. Moreover, high stability increases the predictability of ADCs in clinical use, enabling more precise dose adjustments and treatment management.
Second, non-cleavable linkers significantly reduce off-target toxicity. In traditional ADC designs, cleavable linkers may release part of the drug prematurely in blood or non-target tissues, causing unnecessary side effects. Non-cleavable linkers control drug release so that active metabolites mainly act within target cells. This not only protects healthy tissues from drug damage but also enhances ADC safety, providing patients with a gentler treatment experience.
Finally, non-cleavable linkers enhance the therapeutic index of ADCs. By focusing drug release inside target cells, ADCs achieve a higher efficacy-to-safety ratio. This means that at the same dosage, non-cleavable linker ADCs can exert stronger antitumor effects while reducing systemic toxicity risks. For developing effective and safe next-generation ADCs, this advantage is particularly crucial and is a key consideration for pharmaceutical companies when selecting linker types.
BOC Sciences combines advanced chemistry and deep expertise to support your ADC linker development. From cleavable and non-cleavable linkers to tailored synthesis and complete ADC services, we deliver solutions designed for your project's success.
Non-cleavable linkers come in diverse chemical structures, and their design directly affects ADC stability, drug release efficiency, and therapeutic index. In ADC development, linkers are typically classified as thioether-based, maleimide-based, or other innovative chemistries, depending on their chemical properties and payload type. Each type offers unique advantages, providing flexible options for different ADC drugs.
Thioether-based non-cleavable linkers are among the most widely used. Their core chemical feature is the covalent thioether bond connecting the drug to the antibody, which is extremely stable in plasma and resistant to hydrolysis or enzymatic degradation, ensuring drug integrity in circulation. Common thioether linkers include SMCC (succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate) and MCC (4-(maleimidomethyl)cyclohexane-1-carboxylate), both successfully applied in multiple clinical ADCs. Thioether linkers offer high stability and allow optimization of drug–antibody ratio (DAR), making them a common choice for antitumor ADC design.
Maleimide-based non-cleavable linkers form stable covalent bonds with antibody cysteine residues, achieving efficient drug–antibody conjugation. Maleimide linkers are highly controllable and allow precise DAR adjustment, optimizing ADC pharmacokinetics and therapeutic index. Structurally, maleimide linkers also provide some flexibility, making them suitable for various payload types while balancing stability and drug release efficiency. They represent an important chemical strategy in ADC development.
Beyond thioether and maleimide linkers, various innovative chemistries, such as aryl ether and epoxy propyl-based linkers, have been developed. These can be tailored based on payload chemical properties and target cell characteristics. These linkers improve plasma stability while releasing active drugs via antibody degradation, offering more options for novel ADCs and meeting diverse clinical and research needs.
In ADC development, linker design and selection directly impact drug efficacy, safety, and clinical potential. Due to their high plasma stability and targeted release characteristics, non-cleavable linkers are an essential strategy for optimizing ADCs. However, the type of payload, antibody structure, and therapeutic target all influence linker design and selection. Therefore, careful design of non-cleavable linkers is critical to achieving efficient and safe ADCs.
When choosing non-cleavable linkers, multiple factors must be considered. First, the linker must be chemically compatible with the antibody, stably binding specific residues (e.g., cysteine or lysine) while preserving antibody integrity. Second, plasma stability and lysosomal degradation efficiency must be ensured, preventing premature drug release in circulation while enabling efficient intracellular drug release. Additionally, the DAR impact on efficacy and toxicity should be evaluated, and linker design optimized to maximize payload delivery. Finally, linker chemistry should be selected based on disease type and target cell characteristics to balance efficacy and safety.
The chemical structure of non-cleavable linkers directly influences ADC DAR. Proper linker design enables uniform drug distribution, reduces aggregation, and improves ADC pharmacokinetic stability. For example, both thioether and maleimide linkers allow predictable DAR through controlled reaction sites, ensuring drug activity and minimizing free drug in circulation. Optimized DAR enhances therapeutic effect, reduces side effects, and improves ADC clinical feasibility and competitiveness.
Different payloads have varying linker requirements. Small-molecule microtubule inhibitors usually require highly stable linkers (e.g., thioether) to prevent premature release in plasma, while DNA-damaging agents or protease inhibitors may benefit from aryl ether or other customized non-cleavable linkers to ensure full activity inside target cells. During selection, developers should match payload chemistry, antibody characteristics, and target cell environment for optimal ADC performance. Additionally, structural modifications to non-cleavable linkers can enhance antibody degradation-mediated drug release, improving ADC efficacy.
BOC Sciences offers high-quality custom synthesis services for non-cleavable linkers, focusing on the diverse needs of ADC research and production. Our services cover the full workflow, from initial design and chemical synthesis to structural optimization, ensuring each linker meets high standards in chemical stability, plasma tolerance, and intracellular drug release efficiency.
Non-cleavable linkers have demonstrated broad application value in ADC clinical development, particularly in tumor therapy and novel ADC research. Through high stability, precise release, and reduced off-target toxicity, non-cleavable linkers significantly optimize ADC efficacy, providing safer and more effective treatments for patients.
In cancer therapy, non-cleavable linker ADCs have become a core strategy. For example, T-DM1 (Kadcyla) uses an MCC non-cleavable linker to achieve targeted therapy for HER2-positive breast cancer. The non-cleavable linker ensures the drug is released only inside cancer cells, enhancing antitumor activity while reducing side effects on healthy tissues. The success of these ADCs highlights the reliability and practicality of non-cleavable linkers in clinical oncology drug development.
With continuous advancements in ADC technology, more novel ADC candidates employ non-cleavable linkers. In addition to common solid tumor ADCs, some ADCs targeting hematologic malignancies or immune cells are beginning to leverage non-cleavable linkers to optimize drug release and reduce toxicity. These emerging ADCs have demonstrated promising efficacy and safety in preclinical and early clinical studies, indicating the growing potential of non-cleavable linkers in future ADC development.
Cleavable linkers release drugs via enzymatic activity, pH changes, or reducing conditions, which may risk premature release in circulation. Non-cleavable linkers are chemically stable in plasma and rely on antibody degradation inside target cells for drug release, enhancing ADC stability and safety.
Non-cleavable linkers improve ADC stability by preventing drug release in the bloodstream. The drug is only released when the antibody is degraded inside target cells, reducing systemic toxicity and extending half-life, which ensures predictable pharmacokinetics and safer therapeutic outcomes.
Non-cleavable linkers are chosen for their high plasma stability, reduced off-target toxicity, and controlled intracellular drug release. They enhance the therapeutic index, improve pharmacokinetics, and are ideal for developing safer, more effective ADCs, especially in oncology applications.
No, SMCC (succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate) is a non-cleavable linker. It forms a stable covalent bond between the antibody and payload, ensuring the drug remains attached during circulation and is released primarily through antibody degradation inside target cells.
MCC (4-(maleimidomethyl)cyclohexane-1-carboxylate) is a non-cleavable linker widely used in ADCs. It covalently attaches drugs to antibodies via cysteine residues, providing high plasma stability and controlled intracellular release, reducing off-target toxicity and enhancing therapeutic index.
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.










