BOC Sciences is committed to providing efficient and reliable payload development services for antibody-drug conjugates (ADCs). As one of the most commonly used classes of cytotoxins in ADCs, microtubule inhibitors such as MMAE, MMAF, DM1, and DM4 are widely applied in numerous clinical and investigational ADC programs due to their excellent antitumor activity and mature conjugation characteristics. Leveraging our extensive synthesis experience, diverse structural resources, and modular linker-toxin design capabilities, we offer end-to-end services from structural customization, small-scale synthesis, and process optimization to gram- or kilogram-scale production, ensuring smooth progression through early pharmacodynamic validation and preclinical development. BOC Sciences, driven by technological innovation and rigorous quality control, empowers global ADC development teams to accelerate the creation of next-generation precision therapeutics.
Microtubule inhibitors (tubulin inhibitors) are a class of cytotoxic molecules that disrupt the dynamic balance of microtubules, commonly used as potent cytotoxic payloads in ADCs. These molecules induce cancer cell apoptosis or cell cycle arrest by inhibiting microtubule polymerization or promoting depolymerization, thereby preventing spindle formation during mitosis. Common categories of microtubule inhibitors include Taxanes, Vinca Alkaloids, Dolastatin derivatives, and monomethyl auristatin drugs such as MMAE and MMAF.
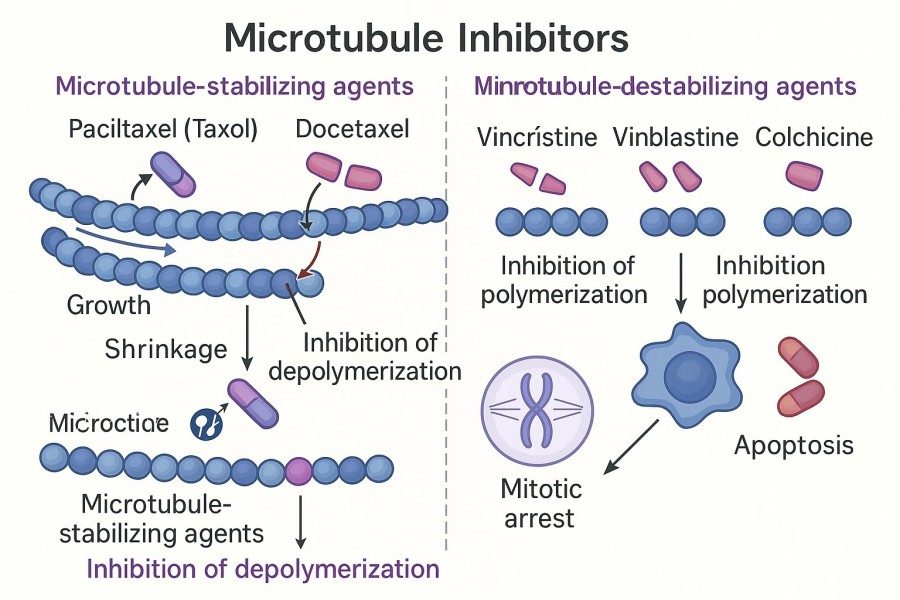 Fig. 1. Microtubule inhibitors examples (BOC Sciences Authorized).
Fig. 1. Microtubule inhibitors examples (BOC Sciences Authorized).
In ADC development, microtubule inhibitors are widely employed due to their high-targeted killing efficiency and irreversible anti-proliferative properties. For instance, MMAE and MMAF have been successfully incorporated into multiple approved ADCs, significantly enhancing tumor-selective cytotoxicity and treatment safety. Beyond ADCs, microtubule inhibitors are also used in chemotherapy drug development, tumor biology studies, cell cycle analysis, and high-throughput screening models, providing key tool molecules for precision anticancer drug design and novel ADC platform development.
Tubulin inhibitors possess complex, highly chiral structures and require stringent reaction conditions. During scale-up, issues such as impurity accumulation and yield reduction often arise. With our advanced multi-step organic synthesis platform and scale-up expertise, we optimize processes and control key steps to achieve high-purity synthesis from milligram to kilogram scales.
Introducing functional groups to tubulin toxins requires precise site-specific modification, as any deviation may compromise biological activity. We provide structural modification simulations and targeted functional group installation services to enable stable and controlled linker integration without disrupting the toxin's core structure.
The strong hydrophobicity of tubulin inhibitors can lead to ADC aggregation and precipitation, compromising drug consistency and clinical safety. BOC Sciences offers hydrophilic modifications for hydrophobic toxins, DAR optimization, and conjugation condition refinement to enhance solubility, stability, and formulation uniformity of ADCs.
Improper linker design can impair intracellular toxin release at the target site, reducing ADC therapeutic efficacy. With extensive experience in synthesizing various linker types, we design cleavable or non-cleavable linkers tailored to specific tubulin inhibitors, ensuring efficient and targeted toxin release.
BOC Sciences is dedicated to delivering comprehensive, professional, and high-quality microtubule inhibitor development services for ADCs, supporting the entire process from early research to preclinical studies. We integrate advanced synthesis technologies, linker chemistry, toxin structure optimization, and a stringent quality control system to create a competitive service offering, which includes:
We maintain a complete library of microtubule inhibitors, covering various natural, semi-synthetic, and fully synthetic cytotoxic structures, including:
Linkers play a key role in controlling ADC pharmacodynamics. BOC Sciences provides multiple conjugation strategies and matching site designs:
With advanced synthesis platforms and conjugation technologies, BOC Sciences provides integrated solutions from toxin development to ADC construction, including:
To ensure smooth project advancement, we are equipped with a comprehensive analytical platform for precise quality monitoring at every critical step.
Toxin and intermediate analysis:
Conjugate analysis:
BOC Sciences has years of expertise in developing ADC toxins and linkers. We have successfully supported multiple international ADC projects covering all stages from early screening to preclinical studies, with mature and reliable technical routes.
We offer a variety of microtubule inhibitor structures including MMAE, MMAF, DM1, DM4, and their derivatives, and support the custom development of innovative toxins with specific functional groups as needed by our clients.
We provide services such as toxin structure modification, linker selection optimization, and linker-toxin module design to ensure structural and functional compatibility of ADCs and enhance project success rates.
From raw materials and intermediates to final toxins and conjugates, we employ HPLC, MS, NMR, and other analytical methods to ensure product purity, activity, and batch-to-batch consistency.
BOC Sciences serves hundreds of biotechnology companies, pharmaceutical enterprises, and research institutions worldwide. Our projects span key markets across North America, Europe, and Asia, earning broad customer trust and long-term partnerships.
We support rapid project initiation, phased delivery, and small-scale trial production. Our team provides technical consultations and development recommendations to help clients advance ADC projects under tight timelines.
We offer products compliant with GLP/GMP standards along with complete documentation support, including CoA, analytical reports, and stability data, facilitating smooth IND or clinical submissions.
Beyond providing toxins and linkers, we offer comprehensive services including ADC conjugation, analytical method development, and biological activity validation—creating a one-stop collaboration model to enhance development efficiency.

At project initiation, we engage in in-depth communication with clients to clarify the desired tubulin inhibitor structure, intended use (screening, conjugation, or preclinical), purity requirements, and batch size. We then formulate a rational synthetic route, process parameters, and delivery schedule to ensure efficient downstream development.
Based on the target molecule, we assess existing literature and databases to design a suitable retrosynthetic pathway. We optimize key intermediates and reaction conditions to improve efficiency and yield, reduce impurities, and ensure the scalability and consistency of the process.
We perform small-scale synthesis in the lab to validate the feasibility and reproducibility of each step. Structural confirmation (NMR, MS), purity analysis (HPLC), and intermediate evaluation are completed to lay a solid foundation for scale-up while identifying potential risk points for optimization.
Under guaranteed quality, reaction scale is gradually increased to gram or higher levels. Stable and efficient reaction and purification processes are used for pilot production. Process control parameters for key steps are established to ensure smooth transition from lab to plant-scale production.
Multi-step chromatographic separation, recrystallization, or membrane separation techniques are employed for in-depth purification of the target toxin, ensuring >98% purity. Comprehensive quality analyses are conducted, including structure confirmation, impurity profiling, and residual solvent detection, to ensure each batch meets delivery standards.

Final products are sealed with internal packaging materials and stored under light-protected/refrigerated conditions to maintain toxin stability and activity. We provide clients with detailed synthetic routes, analytical reports, Certificates of Analysis (CoA), and Safety Data Sheets (SDS) to support R&D or regulatory filing requirements.
Microtubule inhibitors are molecules that disrupt microtubule dynamics and block cell division. In ADCs, they are used as microtubule inhibitor payloads to achieve targeted tumor cell killing. Typical examples include Taxanes (e.g., Docetaxel), Vinca Alkaloids (e.g., Vincristine), Dolastatin derivatives, and MMAE/MMAF, which induce cancer cell apoptosis or cell cycle arrest by inhibiting microtubule polymerization or promoting depolymerization.
Yes, Colchicine is a classic microtubule inhibitor. It blocks microtubule polymerization, preventing spindle formation during mitosis and thereby inhibiting cell proliferation. In ADC development, Colchicine and its derivatives provide mechanistic insights for designing microtubule inhibitor payloads, serving as a foundation for precision-targeted anticancer drug development.
Mitotic inhibitors include various drugs that disrupt microtubule function, such as Taxanes, Vinca Alkaloids, Dolastatin derivatives, and MMAE/MMAF. They suppress cell division by blocking spindle formation or altering microtubule dynamics. In ADCs, these drugs serve as microtubule inhibitor payloads, enabling precise tumor cell killing while reducing systemic toxicity to normal tissues.
We can synthesize a wide range of tubulin inhibitors and their modified versions, including MMAE, MMAF, DM1, DM4, Auristatin derivatives, Maytansinoids, and client-specified structures.
Yes. We can design and synthesize complete linker-toxin modules such as Val-Cit-PABC-MMAE for efficient downstream antibody conjugation.
Absolutely. We support the introduction of functional groups such as carboxylation, amination, and thiolation to accommodate various conjugation strategies.
Yes. We provide toxin synthesis and scale-up services ranging from milligram to gram and kilogram levels, covering all stages from early R&D to preclinical studies.
We provide complete analytical documentation for each batch, including NMR, MS, HPLC spectra, purity reports, and Certificates of Analysis (CoA) to ensure traceability of product quality.
Yes. We accept client-provided target molecules, synthetic routes, and technical parameters, and can optimize the process based on this information to improve yield and purity.
Yes. In addition to toxin synthesis, we provide full ADC construction technical support, including linker selection, conjugation method development, and DAR evaluation.
You can fill out a project request form on our website or contact our technical team directly. We will provide a customized quote and development plan based on your target structure and service requirements.
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










