BOC Sciences is a leading supplier of antibody-drug conjugates (ADCs) products. We offer a wide range of ADC products including Disitamab vedotin, Teisotuzumab vedotin, Polatuzumab vedotin, Tisotumab vedotin, and others. Our ADCs are manufactured using state-of-the-art technology and are of the highest quality and purity. They are available in different formulations, including lyophilized powders, liquids and ready-to-use solutions. Our ADC supply capabilities are flexible and can be customized to meet customers' specific needs and are available in a range of packaging options, including bulk, vials and pre-fill.
As a new type of biological agents, ADCs have great potential in tumor treatment. ADCs deliver cytotoxic payloads directly to the desired site of action to enhance efficacy and minimize off-target effects. Antibody engineering technology can make ADC more uniform and stable, and a new generation of ADC drugs is in clinical trials, which is expected to improve the therapeutic effect and safety. In addition, with the research on more tumor-specific antigen targets and the in-depth understanding of the tumor cytotoxic drug release mechanism, ADCs are showing an explosive development trend in the field of tumor treatment. However, ADC also faces some challenges and constraints in application. The payload may escape or spread outside the cell, causing systemic toxicity and thus affecting therapeutic efficacy and safety. In addition, the process of antibody stability and large-scale production is complex and the preparation cost is high, which also limits its clinical promotion and application. Currently, there are at least dozens of ADC drug candidates undergoing clinical evaluation around the world.
The development of ADCs can be traced back to the early 20th century with the "magic bullet" concept. In the 1900s, Paul Ehrlich proposed the idea of delivering drugs directly to disease sites using targeted molecules, laying the theoretical foundation for ADCs. In the 1980s, with the maturation of monoclonal antibody technology, the first-generation ADC prototypes emerged. However, clinical applications were limited due to unstable linkers and toxicity issues. In 2000, Mylotarg became the first ADC approved by the FDA for treating acute myeloid leukemia, marking the beginning of the clinical era for ADCs. Subsequently, second-generation ADCs such as Adcetris and Kadcyla improved efficacy and safety through enhanced linker and payload designs. In recent years, third-generation ADCs have adopted high drug-to-antibody ratios (DAR), smart release linkers, and novel payloads to achieve more precise and efficient tumor killing. ADC technology continues to evolve as a crucial direction in precision oncology.
The unique advantage of ADCs lies in their mechanism of "precise delivery plus selective cytotoxicity." The process involves four key steps:

ADCs integrate the high targeting specificity of monoclonal antibodies with the potent killing ability of small-molecule drugs, representing the next generation of anticancer therapies. Their core advantages include:
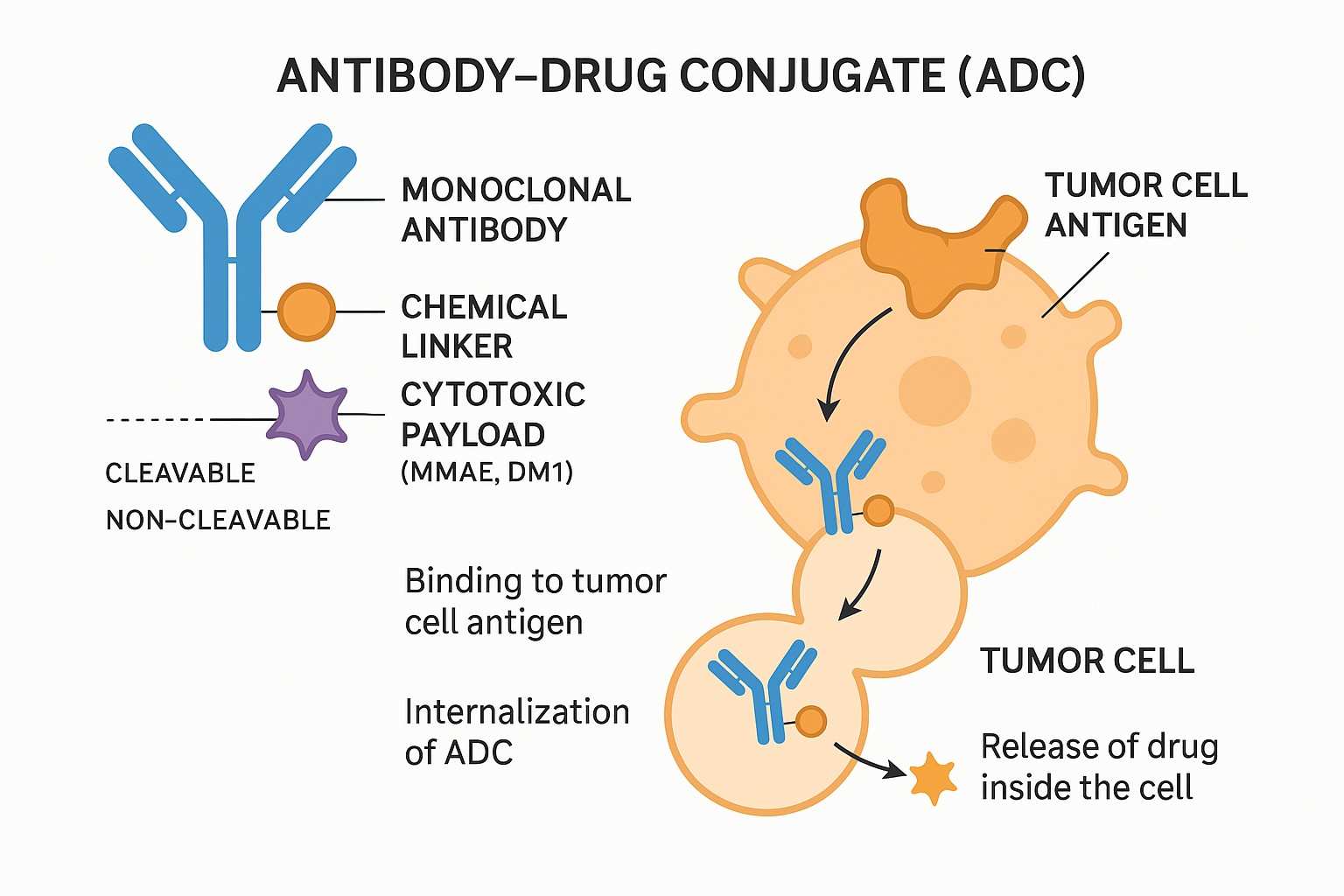
ADCs consist of three core components — the antibody, the linker, and the payload. This modular design endows ADCs with outstanding targeting and cytotoxic capabilities, while offering high flexibility and customization in drug development.
In antibody-drug conjugates (ADCs), the antibody serves as a precise "navigation system," targeting tumor-specific antigens to deliver potent cytotoxins directly to diseased tissues while sparing healthy cells. Effective ADC antibodies require high binding affinity, low immunogenicity through humanization or fully human design, and strong tissue penetration to infiltrate solid tumors. However, the design of a clinically successful ADC depends not only on factors such as the potency of the payload and its ratio, linker stability, and release of the payload, but also on the choice of conjugation technology. Traditional random conjugation often produces heterogeneous ADCs with variable drug loading. Advanced site-specific conjugation technologies now offer improved structural uniformity, pharmacokinetics, and therapeutic consistency, driving next-generation ADC development.
Payload is a key component for ADC to exert its cytotoxic effect and must meet several main requirements: (1) High degree of cytotoxicity, usually with an IC50 value of low nanomolar or picomolar levels (Fig. 1); (2) Clear target and mechanism of action; (3) (potential) chemical attachment site. Maytansine derivatives (DM1/DM4) or Auristatin (MMAE/MMAF) are commonly used microtubule inhibitors. Other types of cytotoxic drugs include enediyne (carleomycin), doxycycline derivatives, pyrrolobenzodiazepine (PBD) and indolobenzodiazepine, all of which target small grooves of DNA. In addition, there are quinoline alkaloids (SN-38), which can inhibit DNA topoisomerase I.
One of the biggest challenges in ADC development is selecting the appropriate linker to conjugate the cytotoxic payload to the antibody. Linker chemistry affects various properties of the ADC, including toxicity, specificity, stability, and potency. Linkers can be broadly classified as cleavable linker (the payload can dissociate from the mAb at the tumor site) or non-cleavable linker (the payload remains bound to the mAb). Common cleavable linkers include chemically active linkers, acid-cleavable linkers, reducible chain linkers and enzyme-cleavable linkers. Non-cleavable linkers release cytotoxic payloads during lysosomal degradation of antibody-drug conjugates in the tumor environment, bypassing nonspecific dispersion of toxic agents.
BOC Sciences leverages cutting-edge technologies and extensive expertise to accelerate your ADC development. From cytotoxins and linkers to custom synthesis and comprehensive ADC services, we are committed to empowering your next breakthrough.
As important representatives of precision targeted therapy, ADCs have seen multiple successful clinical approvals by regulatory agencies worldwide in recent years. Below are several representative examples of ADCs:
Composed of trastuzumab conjugated to the cytotoxin DM1 via a stable thioether linker, used to treat HER2-positive breast cancer patients.
Consists of an anti-CD30 monoclonal antibody linked to the cytotoxin MMAE through a cleavable peptide linker, indicated for Hodgkin lymphoma.
Combines an anti-Trop-2 antibody with the cytotoxin SN-38, primarily used to treat triple-negative breast cancer (TNBC) and urothelial carcinoma.
Anti-FRα antibody conjugated to DM4 via cleavable disulfide linker, for FRα-positive platinum-resistant ovarian cancer.
Anti-TF antibody linked to MMAE through cleavable peptide linker, indicated for recurrent or metastatic cervical cancer.
Combines trastuzumab with the topoisomerase I inhibitor deruxtecan, demonstrating outstanding efficacy in various HER2-expressing solid tumors.
An anti-CD79b antibody conjugated with MMAE, used to treat diffuse large B-cell lymphoma (DLBCL).
Targets the CD33 antigen and is approved for the treatment of acute myeloid leukemia (AML).
ADCs, as an innovative targeted therapy strategy, have become a significant breakthrough in cancer treatment. By effectively combining highly selective monoclonal antibodies with potent cytotoxins, ADCs can precisely identify and kill tumor cells while minimizing damage to normal cells. This technology is particularly suitable for treating refractory and relapsed tumors such as breast cancer, lymphoma, and lung cancer. ADCs not only significantly improve therapeutic outcomes but also reduce chemotherapy-associated systemic toxicity, enhancing patients' quality of life. With continuous development of new payloads, linkers, and antibodies, the prospects for ADC applications in cancer therapy are increasingly promising.
Following the significant clinical success of first-generation ADCs, developers are focused on creating next-generation ADCs to enhance efficacy, reduce side effects, and broaden indications. New-generation ADCs emphasize the development of innovative payloads that are more specific and highly toxic to overcome tumor resistance. At the same time, smart linker designs achieve high stability in circulation and precise release within the tumor microenvironment, improving safety and therapeutic outcomes. Furthermore, the application of bispecific or multispecific antibodies enhances targeting precision, and Fc region engineering strengthens immune effector functions. Delivery systems combining nanotechnology further optimize drug distribution and tumor penetration. Multimodal combination therapies, such as synergy with immune checkpoint inhibitors, are becoming effective approaches to boost antitumor activity. BOC Sciences leverages advanced design and synthesis platforms to support clients in accelerating next-generation ADC innovation and development.
Due to their complex structure, ADCs require critical analytical techniques. Key analyses include precise determination of the DAR, purity and homogeneity assessment, linker stability evaluation, and degradation product monitoring to ensure an appropriate payload amount and controlled release. Additionally, immunogenicity and in vivo pharmacokinetic studies help evaluate safety and efficacy. BOC Sciences possesses advanced analytical instruments and a professional team, integrating multiple techniques such as mass spectrometry and liquid chromatography to provide comprehensive ADC analysis services, supporting clients in developing and manufacturing high-quality, highly stable products.
The DAR indicates the average number of payloads attached to each antibody and is a key quality attribute impacting ADC efficacy and safety. Low DAR reduces potency, while high DAR may alter pharmacokinetics and increase toxicity. Variations in reactant concentrations during coupling often cause DAR heterogeneity, particularly in randomly conjugated ADCs. Thus, precise control of the coupling reaction is crucial. To avoid unconjugated antibodies or DAR variability during production, strict process controls are needed. Common DAR determination techniques include HIC, RPLC, LC-MS, UV/Vis spectroscopy, and CE-SDS, enabling accurate assessment of ADC quality.
Pharmacokinetic (PK) studies of ADCs are critical for assessing their behavior in vivo. Because ADCs combine antibodies with small-molecule drugs, their PK characteristics reflect both the long circulation half-life of antibodies and the influence of payload release and metabolism. Key PK parameters include absorption, distribution, metabolism, and excretion (ADME), as well as the stability and exposure levels of the drug in plasma and tissues. In-depth PK analysis aids in optimizing ADC design, dosing regimens, and administration strategies to maximize efficacy while minimizing toxicity. Common evaluation methods include immunoassays, LC-MS/MS detection, and bioanalytical modeling and prediction.
The manufacturing of ADCs integrates advanced biopharmaceutical and chemical synthesis technologies, demanding extremely high precision and quality control. The entire process covers antibody production, linker and payload synthesis, and conjugation reactions to ensure the final product meets high standards for efficacy, safety, and consistency. Leveraging GMP-grade production environments and mature analytical platforms, BOC Sciences offers clients one-stop ADC manufacturing solutions from process development to commercial production, accelerating clinical and market translation of innovative drugs.
The quality of ADCs depends heavily on the manufacturing process, more so than other APIs. Achieving a uniform product requires a robust and well-controlled process. Key parameters in the coupling step must be thoroughly studied and optimized, while storage and handling should align with stability data of intermediates and ADCs. Successful process development also relies on skilled personnel and advanced equipment. Micro-jacketed vessels allow precise coupling experiments at milligram scale with excellent temperature control, supporting scale-up predictions. However, gram-scale purification runs are necessary to optimize tangential flow filtration (TFF) conditions effectively.
The production of ADC drugs faces major challenges in designing and operating facilities that safely handle highly potent cytotoxins and their residues. This includes safe material transfer, waste disposal, and contamination control. Comprehensive personnel training is critical to ensure proper equipment use and adherence to strict safety protocols. A robust personal protective equipment (PPE) program must address residual risks not mitigated by engineering controls, alongside contingency plans for toxic releases. Cleaning validation also demands highly sensitive detection techniques, as conventional TOC or HPLC methods often lack sufficient sensitivity. Validated ELISA or LC-MS methods are often required for residual cytotoxin detection.
Purification of ADCs is a critical step to ensure their safety, efficacy, and stability. Due to the complex molecular structure of ADCs, which combine large antibody components with small-molecule cytotoxic drugs, the purification process must remove unconjugated free drug, reaction byproducts, and other impurities while preserving antibody activity and conjugation efficiency. Common purification techniques include chromatography methods such as Protein A affinity, ion exchange, and hydrophobic interaction chromatography, as well as tangential flow filtration (TFF). By optimizing purification parameters, high purity, high recovery, and consistency can be achieved, laying a solid foundation for subsequent quality control and clinical application.
Although ADCs show tremendous potential in cancer treatment, their development and application still face many challenges. Firstly, the complex structure makes the manufacturing process highly dependent on advanced technologies and stringent quality control to ensure batch-to-batch consistency. Secondly, linker stability and controlled payload release are critical determinants of ADC safety and efficacy; any deviation may lead to increased toxicity or reduced therapeutic effect. Moreover, tumor heterogeneity and variable antigen expression can affect targeting precision, limiting therapeutic applicability. Complex pharmacokinetics, immunogenicity, and resistance issues also pose obstacles in clinical development. BOC Sciences is committed to technological innovation and process optimization to help clients overcome these challenges and accelerate the development and commercialization of high-quality ADC drugs.
An antibody drug conjugate (ADC) is a targeted therapy that uses monoclonal antibodies to deliver highly potent cytotoxic drugs specifically to certain tissues or cells. This targeted delivery increases therapeutic effectiveness while reducing damage to healthy tissues, minimizing side effects commonly seen with conventional chemotherapy.
The antibody drug conjugate market is experiencing rapid growth, driven by advances in targeted therapies and precision medicine. It is projected to maintain a double-digit compound annual growth rate (CAGR) over the coming years, with expanding applications especially in oncology immunotherapy and treatment of various cancers.
Manufacturing an ADC involves several critical steps: selection and preparation of a suitable monoclonal antibody, synthesis of a potent cytotoxic drug, designing and producing a stable chemical linker, conjugation of the payload to the antibody, followed by rigorous purification and quality control to ensure product efficacy and safety.
An Anti-Trop-2 ADC specifically targets the Trop-2 protein found on the surface of many tumor cells. By binding to this antigen, the ADC delivers cytotoxic drugs directly into cancer cells, making it an effective treatment option for various solid tumors such as breast, lung, and other epithelial cancers.
The ADC binds to specific antigens on the surface of target cells through its antibody component. This binding triggers cellular processes that internalize the ADC via endocytosis, allowing the conjugated cytotoxic drug to be released inside the cell, where it exerts its tumor-killing effect.
ADC characterization involves techniques like mass spectrometry, high-performance liquid chromatography (HPLC), SDS-PAGE, and binding activity assays. These methods assess the drug-to-antibody ratio (DAR), purity, structural integrity, stability, and functional activity to ensure the ADC meets quality standards.
The ADC development pipeline comprises candidate molecules undergoing preclinical research, followed by multiple phases of clinical trials (Phase I, II, III), and ultimately marketed products. This pipeline reflects the extensive ongoing research, innovation, and clinical validation efforts in this rapidly evolving therapeutic area.
An ADC is composed of three main parts: a monoclonal antibody that specifically recognizes target cells, a chemical linker that connects the antibody to the drug, and a cytotoxic payload. The linker's stability is crucial to ensure the drug is only released inside the targeted cell, enhancing treatment precision.
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.










