As a new generation of ADC payloads, RNA polymerase inhibitor toxins are paving the way for the treatment of refractory and drug-resistant tumors due to their unique transcription inhibition mechanism and potent cytotoxicity. BOC Sciences has been deeply engaged in the ADC field for years. Leveraging its robust synthesis platform, conjugation technologies, and analytical capabilities, we provide end-to-end services from custom synthesis of RNA polymerase inhibitor molecules to conjugate construction and analysis, assisting partners in advancing ADC candidates from concept to product.
RNA polymerase inhibitors are molecules that specifically suppress RNA polymerase activity, blocking RNA transcription and gene expression. In ADCs, these inhibitors can serve as potent cytotoxic payloads, precisely interfering with transcription in tumor cells to induce cell cycle arrest and apoptosis. Common categories include nucleoside analogs, non-nucleoside small-molecule inhibitors, and natural product derivatives. Their mechanisms typically involve blocking RNA chain synthesis, competitively inhibiting enzyme activity, or altering polymerase conformation, effectively suppressing RNA transcription and protein synthesis in cancer cells for targeted killing.
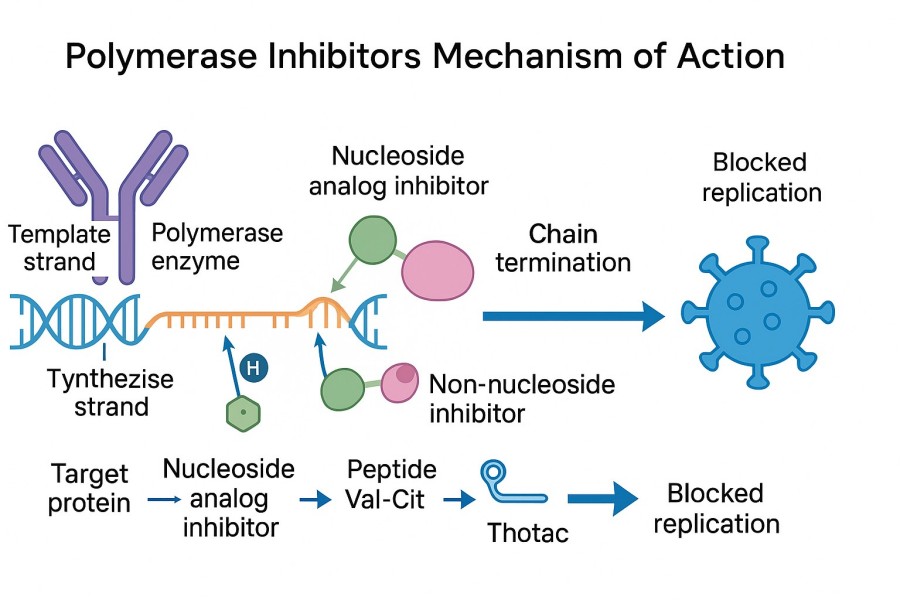 Fig. 1. Polymerase inhibitors mechanism of action (BOC Sciences Authorized).
Fig. 1. Polymerase inhibitors mechanism of action (BOC Sciences Authorized).
In ADC development, RNA polymerase inhibitors are widely applied due to their high selectivity and controllability, enhancing ADC antitumor activity and safety. They are also used in tumor biology research, gene expression studies, high-throughput drug screening platforms, and novel ADC payload development, providing essential tool molecules for precision medicine and innovative anticancer drug design.
RNA polymerase inhibitors are difficult to extract from natural sources and have complex structures. We offer efficient semi-synthesis and total synthesis processes to prepare high-purity α-Amanitin and its derivatives in bulk, supporting custom services from milligram to gram scale.
These toxins are highly potent, posing risks of systemic exposure. We enhance ADC safety and therapeutic index through linker screening and site-specific conjugation technologies to ensure toxin release specifically at the target site.
RNA polymerase inhibitors possess limited functional groups, posing conjugation difficulties. We have developed a variety of dedicated linker structures and modification techniques to achieve efficient and directional conjugation, ensuring ADC stability and activity.
The complex structure of the toxins makes analysis technically demanding. Our comprehensive analytical platform employs LC-MS/MS, SEC, HIC, and other methods to ensure accurate determination of structure, DAR, and purity.
BOC Sciences offers one-stop development services for RNA polymerase inhibitor-based ADC payloads to pharmaceutical companies, research institutes, and ADC startups. Our services cover all stages from toxin synthesis, linker screening, conjugation strategy development, to analysis and scale-up manufacturing. Service modules include:
We provide the following custom services:
Given the high polarity and complex structures of RNA polymerase inhibitors, linker strategies play a critical role in release control and toxicity regulation. We offer:
By optimizing conjugation sites, controlling drug-to-antibody ratio (DAR), and improving grafting efficiency, we construct highly homogeneous conjugation systems:
We possess complete analytical and quality evaluation capabilities to ensure the uniformity, safety, and traceability of each ADC batch:
We have developed efficient synthesis and modification routes for dozens of natural product toxins, including cyclic peptides, glycosides, amides, flavonoids, and other complex structures, enabling both large-scale production and customization.
We have built a database of over 200 linker chemical scaffolds, allowing rapid screening of controlled-release strategies applicable to RNA polymerase inhibitors, with fast validation services available.
Our company is equipped with advanced bioconjugation facilities and a GMP-compatible pilot platform, enabling seamless transition from microgram-level studies to kilogram-scale production.
We provide full lifecycle CMC technical support for ADC projects, covering structure identification, stability evaluation, formulation development, and quality standard establishment to ensure regulatory compliance of toxin-antibody products.
Our team brings together expertise in organic chemistry, bioengineering, analytical sciences, and formulation development, enabling cross-disciplinary integration from structural design to functional validation.
With flexible project management and modular service systems, we respond quickly to client needs, offering highly customized services including toxin screening, structural modification, and conjugation strategy optimization.

We begin by engaging in in-depth communication with clients to understand their research goals and target characteristics. We clarify the direction of ADC construction, the required type of payload, and linker strategy. Meanwhile, we assess the suitability, safety, and development feasibility of RNA polymerase inhibitors, and formulate an initial project plan.
Based on client needs or target characteristics, we provide natural α-Amanitin purification, derivative design, functional group introduction, and structural construction of novel RNA polymerase inhibitors. We support customized synthesis and structural optimization from milligram to gram scale.
Taking into account the physicochemical properties of the payload and the desired release environment (e.g., pH, enzymes), we design and screen suitable linkers such as Cathepsin B-sensitive peptides, acid-labile bonds, or reducible disulfide bonds to ensure accurate release and stable delivery of the toxin in vivo.
According to the antibody type and linker structure, we develop the optimal conjugation strategy, control the drug-to-antibody ratio (DAR), maintain antibody activity, and improve conjugation efficiency and product uniformity to construct high-quality ADCs.
We use LC-MS/MS, HIC, SEC, and other analytical techniques for structure confirmation, DAR determination, purity analysis, and free toxin residue detection of ADCs. In vitro release tests and cytotoxicity pre-screening are also conducted to verify the effectiveness and stability of the constructed system.

After experimental verification, BOC Sciences provides pilot-scale production services, establishing scale-up process parameters, batch consistency assessment, formulation stability testing, and delivering complete data packages to support client submission, transfer, or subsequent development.
Polymerase inhibitor drugs include nucleoside analogs, non-nucleoside small-molecule inhibitors, and natural product derivatives. Nucleoside analogs such as Fludarabine and Cytarabine incorporate into RNA chains to block transcription; non-nucleoside small molecules like Rifampicin derivatives directly inhibit polymerase activity; and natural products such as α-amanitin act by altering enzyme conformation. In ADC development, these drugs can be designed as transcription inhibitor payloads to achieve tumor-selective killing and improved therapeutic efficacy.
Some antibiotics inhibit RNA polymerase, thereby blocking bacterial RNA synthesis. For example, Rifampicin binds RNA polymerase to inhibit transcription elongation, Streptolydigin alters polymerase conformation to suppress elongation, and Myxopyronin blocks the formation of the transcription initiation complex. These mechanisms provide important references for designing RNA polymerase inhibitor payloads in ADCs, enabling precise interference with target cell RNA synthesis for antitumor effects.
RNA inhibitors act through multiple mechanisms, including directly blocking RNA polymerase activity to prevent chain elongation, competitively inhibiting substrate binding at the active site, and altering enzyme conformation or disrupting transcription complex formation to block initiation or elongation. In ADC applications, RNA inhibitors as transcription inhibitor payloads can efficiently and precisely interfere with RNA synthesis in cancer cells, enabling targeted killing while reducing systemic toxicity to normal cells, supporting anticancer drug development and precision therapy.
We combine semi-synthetic and total synthesis routes to ensure stable and scalable preparation of high-purity α-Amanitin and its functionalized derivatives, meeting demands from R&D to clinical stages.
Yes. We can perform functional group modifications at sites such as carboxyl or amino groups without compromising cytotoxic activity, creating derivatives suitable for linkers and improving conjugation efficiency and carrier compatibility.
Compounds like α-Amanitin may carry hepatotoxicity risks. We mitigate systemic toxicity risks during the design phase through targeted antibody selection, linker-controlled release, and toxin dose optimization.
Absolutely. We offer multiple linker platforms (acid-sensitive, enzyme-sensitive, reducible linkers) and can customize linker strategies based on antibody structure and toxin properties to ensure conjugation efficiency and biological activity.
Yes. We precisely control the DAR during conjugation to balance toxicity release and therapeutic efficacy, and also provide DAR analysis services (e.g., HIC, LC-MS) to ensure product quality.
Yes. We have pilot-scale synthesis and purification capabilities, along with cGMP-compliant laboratories, to support preparation, stability studies, and documentation for preclinical submission.
Certainly. Our service modules are flexible—clients can choose single or combined services such as toxin customization, linker development, conjugation optimization, and analytical services according to their project stage and needs.
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










