Non-cleavable linkers are a class of highly stable linkers, designed chemically to resist degradation by enzymes or pH changes in the bloodstream and extracellular environment. The drug is only released in its active form when the ADC is internalized by the target cell and enters the lysosome, where the antibody protein itself is degraded. Leveraging extensive experience in linker chemistry and advanced synthesis platforms, BOC Sciences provides global clients with customized development, optimization, and production services for non-cleavable linkers. We offer a variety of scales, purity levels, and delivery timelines to meet your research needs. We ensure that during linker purification and at every step of quality control (QC), each custom linker product undergoes multiple quality checks using mass spectrometry (MS) and high-performance liquid chromatography (HPLC) analyses.
Non-cleavable linkers require the complete degradation of antibodies in lysosomes to release payloads, and their cleavages are more stable in plasma than cleavable linkers. Furthermore, cytotoxins are not released outside the target cell, reducing its toxicity toward healthy cells. Despite limited bystander effects, cleavage resistance outside of target cells may increase the specificity of drug release. Several in vivo studies and clinical data, for example, have demonstrated that non-cleavable linked ADCs outperform their cleavable counterparts in vivo. Numerous non-cleavable linkers have been explored in ADC development, the most representative being SMCC. These linkers are used in hematological tumors and cancers with high antigen expression.
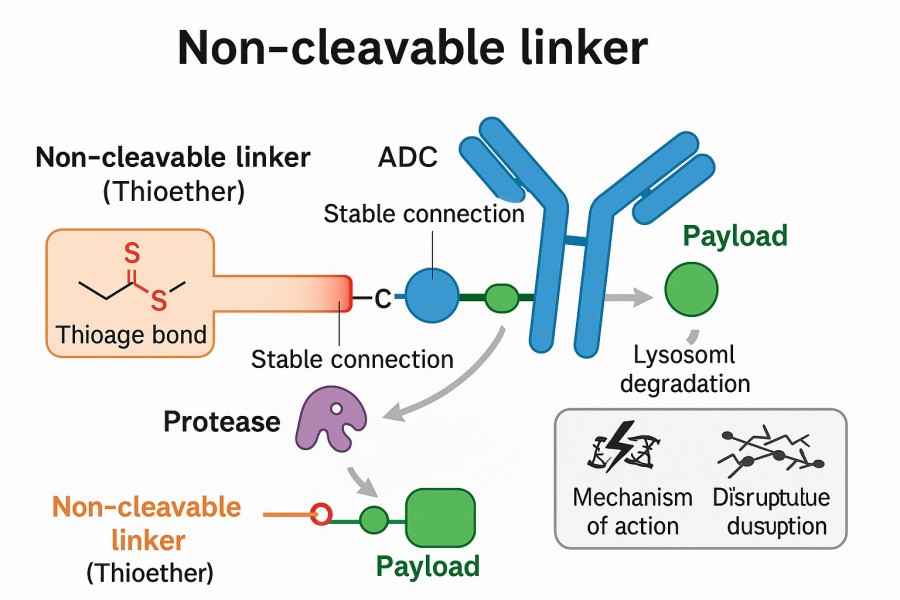 Fig. 1. Non-cleavable linker in ADC (BOC Sciences Authorized).
Fig. 1. Non-cleavable linker in ADC (BOC Sciences Authorized).
Compared to cleavable linkers, the greatest advantage of non-cleavable linkers involved increased plasma stability. Non-cleavable linked ADCs have outperformed the cleavable counterparts in vivo, and mAb degradation within the lysosome after ADC internalization is required for active drug releases from non-cleavable linkers. Potentially, non-cleavable linkers can provide a greater therapeutic window than cleavable linkers because payload derivatives from non-cleavable ADCs can eliminate target cells effectively. In addition, a potentially reduced off-target toxicity is expected compared to the cleavable linker conjugates because non-cleavable ADCs support greater stability and tolerability.
With years of experience in linker chemistry, BOC Sciences provides global clients with a variety of mature and customizable non-cleavable linker types, including thioether-based, amide-based, and other optimized stable scaffolds. Through scientific linker selection and precise conjugation strategies, we help clients achieve efficient drug release and targeted killing while maintaining ADC stability, significantly enhancing the clinical translation potential of ADC candidates.
Several non-cleavable alkyl and polymeric linkers have been explored in ADC development. A notable example is MCC, which couples the payload with the antibody via a thioether bond. Furthermore, this linker is especially useful as the cyclohexane ring provides steric hindrance that reduces hydrolysis of the resulting thioether. With years of ADC research, development, and production capabilities, BOC Sciences' scientists can select and design the most appropriate payload structure combined with a non-cleavable linker to maximize anti-tumor efficacy.
Amide linkers are non-cleavable, highly stable linkers. After being internalized by tumor cells, the ADC antibody is degraded in the lysosome, releasing the small-molecule cytotoxin attached via the amide linker to exert its anti-tumor effects. Stable amide bonds are increasingly used to improve the stability and tolerability of ADC drugs in both the bloodstream and intracellular environment. BOC Sciences provides a one-stop platform for small-molecule ADC development and manufacturing, from preclinical to commercial stages. We offer comprehensive services, including synthetic development, process development, ADC analysis and characterization, and commercial-scale production.
Leveraging extensive experience in linker chemistry and conjugation, BOC Sciences provides full-process support from molecular design to process scale-up. Our strategies cover key aspects such as linker structure optimization, antibody modification and conjugation, drug compatibility studies, chemical stability validation, comprehensive analytical characterization, and process development, ensuring that each step is scientifically sound, controllable, and traceable. Through this systematic design and implementation approach, we help clients maximize ADC safety and efficacy while accelerating clinical translation of drug candidates. Our design services include:
With deep expertise in linker R&D, we are familiar with various non-cleavable linkers such as thioether and amide linkers, ensuring rational structural design and excellent stability.
We provide full-process services from linker design, synthesis, antibody conjugation, analytical characterization to process scale-up, helping clients efficiently advance ADC projects and shorten R&D timelines.
Tailored to different antibody structures, drug molecule characteristics, and R&D goals, we offer flexible custom services, including site-specific modification, linker arm length optimization, and drug compatibility assessment.
Using advanced instruments such as LC-MS, NMR, and HPLC, we precisely characterize linker structure, conjugation efficiency, and DAR, ensuring reliable data and controllable results.
All experiments and production comply with cGMP standards, suitable for early research, preclinical development, and clinical stages, ensuring product quality and regulatory compliance.
Our professional team serves clients worldwide, providing flexible project management and technical support to ensure non-cleavable linker solutions meet diverse research and commercialization needs.

We gain a deep understanding of clients' research objectives, drug physicochemical properties, and antibody characteristics, and perform a scientific assessment based on the project background, laying a solid foundation for subsequent linker design.
Based on the assessment, we provide multiple non-cleavable linker design options, covering different chemical structures and synthetic routes, allowing clients to select the optimal solution flexibly.
Small-scale experiments are conducted to verify linker stability and conjugation efficiency, continuously optimizing drug activity and DAR to ensure overall performance meets R&D requirements.
Small-scale results are translated into pilot and large-scale production processes, achieving high reproducibility and stability in linker synthesis, supporting subsequent clinical advancement.
Using analytical platforms such as LC-MS, NMR, and HPLC, we perform comprehensive testing of linkers and ADCs, ensuring structural clarity, reliable purity, and regulatory-compliant quality.

Clients receive complete experimental data and quality reports to meet registration and compliance requirements, along with continued R&D and technical support services.
The main reason for choosing a non-cleavable linker is its extremely high serum stability. The linker does not release the cytotoxic drug prematurely in vivo; the drug is only activated once the antibody enters the target cell and is degraded. This feature effectively reduces off-target toxicity and enhances the therapeutic window of ADCs, making it widely used in drug development requiring precise control of drug activity.
Common strategies include forming thioether bonds through cysteine modification or amide bonds through lysine modification. These chemical structures provide strong hydrolytic and enzymatic resistance. Additionally, linker arm length and structure are adjusted according to drug solubility and antibody binding sites to optimize ADC pharmacokinetics and pharmacodynamics.
Mechanistically, non-cleavable linkers are resistant to proteolytic degradation and usually rely on full degradation of the antibody to release attached linker-payload complexes. This mechanism requires the payload to remain active while linker bound. For example, MMAE is a protein-based anti-mitotic drug and most potent in its native form; therefore, it is poorly suited for derivitization with a non-cleavable linker. On the other hand, MMAF retained its potency even when linked with a simple alkyl chain in vitro and in vivo. Due to this, non-cleavable linkers were proposed as a strategy to overcome drug resistance since the linker-payload complex is no longer the substrate for multiple drug resistance. Therefore, the proposed action mechanism of the ADC can be a determinant for linker choice.
BOC Sciences has extensive experience in developing non-cleavable linkers and can design and synthesize various structures, including thioether and amide bonds. We customize linkers of different lengths and functional groups based on antibody type, drug characteristics, and R&D goals to ensure optimal drug conjugation efficiency and stability.
BOC Sciences offers integrated services covering design, synthesis, conjugation, analytical characterization, and process scale-up, ensuring efficient advancement of ADC projects. Leveraging advanced platforms such as LC-MS, HPLC, and NMR, we deliver high-purity linkers with controllable DAR. All services follow cGMP standards, supporting early research through clinical submission stages, ensuring scientific rigor and compliance.
Clients only need to provide basic project requirements, including the target antibody, type of cytotoxic drug, and R&D objectives. BOC Sciences' professional team will perform assessment and design feasible linker strategies, conduct small-scale validation and optimization, and ultimately complete large-scale production. The entire process is transparent and controlled, with complete quality analysis reports to support IND submission and subsequent R&D.
Background
A biopharmaceutical R&D team based in Munich, Germany, was developing an antibody-drug conjugate (ADC) targeting HER2-positive breast cancer. In their earlier development, the team used enzyme-cleavable linkers but found premature drug release in plasma during preclinical pharmacokinetic studies, resulting in increased systemic toxicity and limited efficacy. The team urgently needed a solution to significantly enhance ADC stability in circulation.
How BOC Sciences Helped
Upon receiving the project request, BOC Sciences first assembled an expert team to perform a comprehensive analysis of the target antibody, drug molecule, and clinical indication. Based on the physicochemical properties of the drug's active moieties, we recommended a non-cleavable linker strategy and designed multiple candidate chemical structures.
Key Results
The Publications section highlights scientific articles published by global clients based on our products and services, covering drug development, chemical synthesis, analytical testing, and more, demonstrating the research impact and practical application value.

"During our preclinical ADC development, we needed a highly stable non-cleavable linker with precise DAR control. BOC Sciences provided customized design and synthesis solutions on time. Their professionalism and technical expertise are impressive."
— Dr. James Carter, Senior Research Scientist (USA)
"We faced challenges optimizing a Thioether linker for our ADC candidate. BOC Sciences delivered a robust design, performed detailed analytical characterization, and ensured reproducible synthesis. Their support greatly accelerated our project."
— Ms. Emma Johansson, ADC Project Manager (Sweden)
"BOC Sciences helped us implement a Lys-Amide non-cleavable linker with exceptional stability for our targeted therapy. Their team offered in-depth guidance, timely updates, and high-quality deliverables, exceeding our expectations."
— Dr. Oliver Schmidt, Bioconjugation Scientist (Germany)
"Working with BOC Sciences was a smooth experience. They provided end-to-end non-cleavable linker solutions, including design, synthesis, and analytical verification, allowing our team to focus on ADC efficacy studies. Highly reliable partner."
— Ms. Claire Thompson, Senior Scientist (UK)
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










