Thio-engineered antibody is a modified antibody created through genetic engineering to introduce reactive thiol groups (-SH) at specific positions. Compared to conventional randomly conjugated antibodies, thio-engineered antibodies provide highly uniform, site-specific conjugation sites, significantly enhancing the efficacy and stability of antibody-drug conjugates (ADCs). In modern antibody drug development, precise control of drug-to-antibody ratio (DAR) and conjugation sites is critical for ADC success. BOC Sciences' thio-engineered antibody services meet stringent ADC development requirements, offering advantages such as site-specific conjugation, uniformity control, antibody function retention, and scalable production. Our services cover antibody design, engineered expression, thiol activation, drug conjugation, purification and characterization, as well as downstream application support, providing a complete one-stop solution.
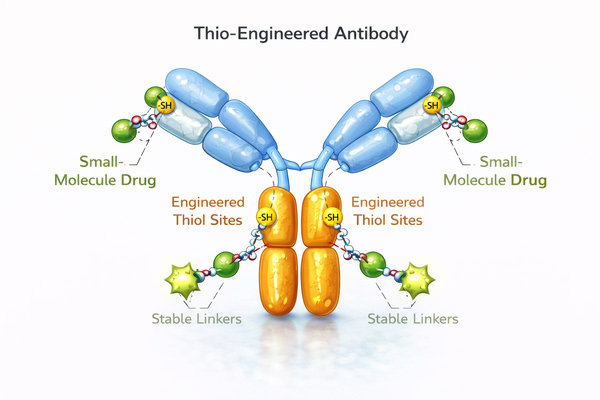
Thio-engineered antibody is a type of modified antibody in which reactive thiol groups (-SH) are introduced at specific positions through genetic engineering. Unlike natural cysteines in antibodies, these artificially designed thiols serve as precisely controllable conjugation sites for stable thioether bond formation with small-molecule drugs, fluorescent probes, or other chemical modifications, enabling site-specific conjugation. The core principle involves selectively introducing cysteine residues in non-critical regions of the Fc or Fab domain to ensure the conjugation sites are chemically reactive without affecting antigen-binding or Fc functions. This strategy allows precise control of drug or label attachment, uniform DAR, and preservation of the antibody's natural folding and stability. Compared to conventional randomly conjugated antibodies, thio-engineered antibodies have more stable conjugation sites, significantly reducing the risk of premature drug release in circulation and improving ADC pharmacokinetics and therapeutic performance.
BOC Sciences provides thio-engineered antibody services using advanced antibody engineering technologies to introduce reactive thiols at specific sites, achieving site-specific conjugation and controlled DAR. We deliver precise, highly uniform conjugated antibodies while maintaining antibody functionality and circulation stability. Whether for single-drug ADCs, multifunctional antibodies, or labeled conjugates, BOC Sciences offers customized solutions to help clients achieve high-quality, efficient antibody drug development.
Precisely designed thiol sites enable stable antibody-drug linkage, controllable DAR, and reduced heterogeneity or functional loss, ensuring consistent ADC stability and efficacy in vivo.
Thiol introduction is validated by structural analysis and experiments, preserving antigen-binding and Fc functions, maintaining natural bioactivity and immunomodulatory effects, providing a solid foundation for high-performance ADCs.
Thioether bonds formed between antibody and drug remain stable in circulation, reducing premature drug release risk, improving ADC pharmacokinetics, and enhancing clinical safety and effectiveness.
Engineered antibody expression and chemical conjugation processes are strictly controlled, minimizing batch variations and ensuring product uniformity and reproducibility, supporting large-scale production and preclinical development.
Experienced biochemistry and antibody engineering teams provide full support from antibody design, expression, thiol optimization, conjugation, to purification and characterization, ensuring efficient R&D and customized service experience.
Equipped with modern laboratories and production facilities, alongside professional technical teams, we offer experimental design, technical consultation, and tailored development support, ensuring efficient R&D and actionable results.

Discuss antibody type, drug choice, and conjugation goals with clients. Develop a personalized thio-engineered antibody plan based on R&D and clinical application needs to ensure feasibility and efficiency.
Select thiol introduction sites via molecular modeling and structural analysis. Construct engineered antibody genes in heavy or light chains using site-directed mutagenesis to maintain folding stability and antigen-binding activity, laying a foundation for high-quality conjugation.
Efficiently express engineered antibodies and purify with Protein A/G or affinity columns, preserving thiol activity, yielding high-purity, structurally intact thio-engineered antibodies for subsequent conjugation.
Protect and activate introduced thiols, optimize buffer and chemical environment, prevent non-specific disulfide formation, and provide an efficient, controllable interface for site-specific drug or label conjugation.
Conjugate drugs such as MMAE, DM1, PBD, or fluorescent/radioactive labels to thiols under mild conditions using maleimide or other linkers, achieving uniform DAR and precise conjugation while preserving antibody function and stability.

Purify conjugates via HIC-HPLC, SEC, or mass spectrometry, analyze DAR, antibody integrity, and stability, and provide complete technical reports and application guidance to support clients' preclinical research and downstream development.
By providing site-specific thiol conjugation, thio-engineered antibodies ensure precise DAR control and uniform drug distribution. This reduces heterogeneity, maintains antibody folding and activity, and enhances pharmacokinetics and in vivo stability, resulting in safer and more effective antibody-drug conjugates.
Thiol residues are usually introduced in non-critical regions of the antibody’s Fc or Fab domains. These sites are carefully selected to avoid affecting antigen binding or Fc-mediated immune functions, ensuring that antibody structure, stability, and biological activity are fully preserved.
BOC Sciences supports conjugation of cytotoxic drugs like MMAE, DM1, and PBD, as well as fluorescent or radiolabel probes. The site-specific thiols allow flexible and controlled attachment, supporting single-drug ADCs, dual-function therapeutic probes, or multi-functional antibody conjugates.
Conjugation efficiency is maximized by activating and protecting thiols under optimized conditions, controlling molar ratios, temperature, and reaction time. HIC-HPLC, SEC, and mass spectrometry are used to confirm uniform DAR, proper antibody folding, and product stability.
Yes. BOC Sciences offers fully customized services, including antibody engineering, thiol design, conjugation strategies, and analytical support. We tailor solutions to specific ADC, dual-label, or multi-functional projects, ensuring high-quality, consistent products that meet research or preclinical requirements.
Thio-engineered antibodies provide site-specific conjugation, precise DAR control, preserved antibody function, improved chemical stability, and reduced heterogeneity. Combined with BOC Sciences’ technical expertise and full-service support, they accelerate ADC development and enhance therapeutic safety and efficacy.
Background
A German biopharmaceutical company was developing a novel ADC targeting HER2-overexpressing breast cancer. The project faced critical technical challenges: conventional random conjugation led to uneven DAR distribution, partial loss of antibody activity, and suboptimal in vivo stability and pharmacokinetics for preclinical studies. The company aimed to use site-specific conjugation technology to improve ADC uniformity and safety, thereby accelerating the candidate drug development process.
How BOC Sciences Helped
BOC Sciences provided a comprehensive thio-engineered antibody conjugation service, including antibody engineering, thiol introduction, drug conjugation, and purification and characterization. Our team collaborated closely with the client, using structural modeling and high-throughput screening to optimize thiol sites, ensuring antigen-binding and Fc functions were fully preserved during conjugation. Precise control of drug loading enabled uniform DAR, improving ADC reproducibility and in vivo stability.
Implementation
Results
Browse BOC Sciences' publications to explore articles from research teams worldwide, showcasing the scientific contributions of our products and services in cutting-edge drug development.

"Developing our ADC candidate required precise site-specific conjugation with strict control over drug-antibody ratios. BOC Sciences provided excellent technical guidance and delivered highly uniform conjugates, meeting our preclinical research timeline perfectly."
— Dr. Emily Johnson, Senior Scientist (USA)
"We needed high-quality antibody-drug conjugates for our oncology research with tight reproducibility standards. BOC Sciences consistently delivered well-characterized products and detailed analytical reports. Their professional support was invaluable throughout the project."
— Mr. Michael Turner, Research Director (UK)
"Facing a complex dual-label conjugation project, BOC Sciences demonstrated exceptional expertise. They provided tailored solutions, optimized conjugation protocols, and ensured consistent product quality. Their responsiveness and technical advice made the collaboration highly successful."
— Dr. Laura Schmidt, Principal Investigator (Germany)
"Our preclinical ADC development demanded precise conjugation and reliable analytical characterization. BOC Sciences exceeded our expectations, delivering high-purity conjugates with comprehensive data packages, while maintaining tight project timelines. Truly a dependable partner."
— Mr. David Clark, Senior Research Scientist (France)
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










