Antibody-drug conjugates (ADCs), as an important vehicle for precision therapy, have become a research hotspot in the fields of oncology and other diseases. The successful development of ADCs relies heavily on high-performance linker and cytotoxin conjugation technologies. Leveraging years of experience in biochemical synthesis and process development, BOC Sciences offers one-stop ADC linker and cytotoxin conjugation services, covering the entire process from custom synthesis to conjugation optimization, helping clients accelerate the development of high-quality ADC products.
ADCs treatment is shifting from hematologic malignancies (lymphoma and leukemia) to solid tumors (breast cancer, urethral cancer, lung cancer, ovarian cancer, etc.), and the expansion of these clinical indications highlights the therapeutic potential of ADCs. During ADC drug construction, the types of antibodies used are unlimited, but the types of linkers, conjugation methods, and toxins are limited. Moreover, the specific combinations of linkers and cytotoxins are most likely to differentiate the efficacy of ADC drugs and obtain patent protection. At BOC Sciences, we provide cost-effective and high-quality custom ADC conjugation services using an integrated approach with various payloads, linkers, and conjugation methods.
BOC Sciences has accumulated mature technical platforms and extensive experience in multiple mainstream ADC technology routes, capable of providing customized linker conjugation services for different payloads, assisting clients in developing ADCs with differentiated advantages.
Calicheamicin-class cytotoxins, with their extremely high cytotoxicity (mechanism of DNA double-strand breakage), were core effective components of the first approved ADCs (e.g., Mylotarg). BOC Sciences can design and synthesize acid-sensitive hydrazone linkers and disulfide linkers suitable for calicheamicin, ensuring drug stability in plasma and efficient release inside target cells. We employ advanced conjugation strategies to control DAR values, optimize linkage stability and homogeneity, providing high-purity and batch-consistent preparation services for calicheamicin-ADCs.
Deruxtecan-class payloads (topoisomerase I inhibitors), due to their strong cell permeability and bystander effect, are widely used in next-generation ADCs such as Enhertu (DS-8201). BOC Sciences offers ester bond and peptide-cleavable linker designs to achieve controlled release of deruxtecan while enhancing ADC efficacy and safety. We support high DAR conjugations and reduce aggregation issues caused by hydrophobicity, ensuring conjugates with excellent pharmacokinetic properties.
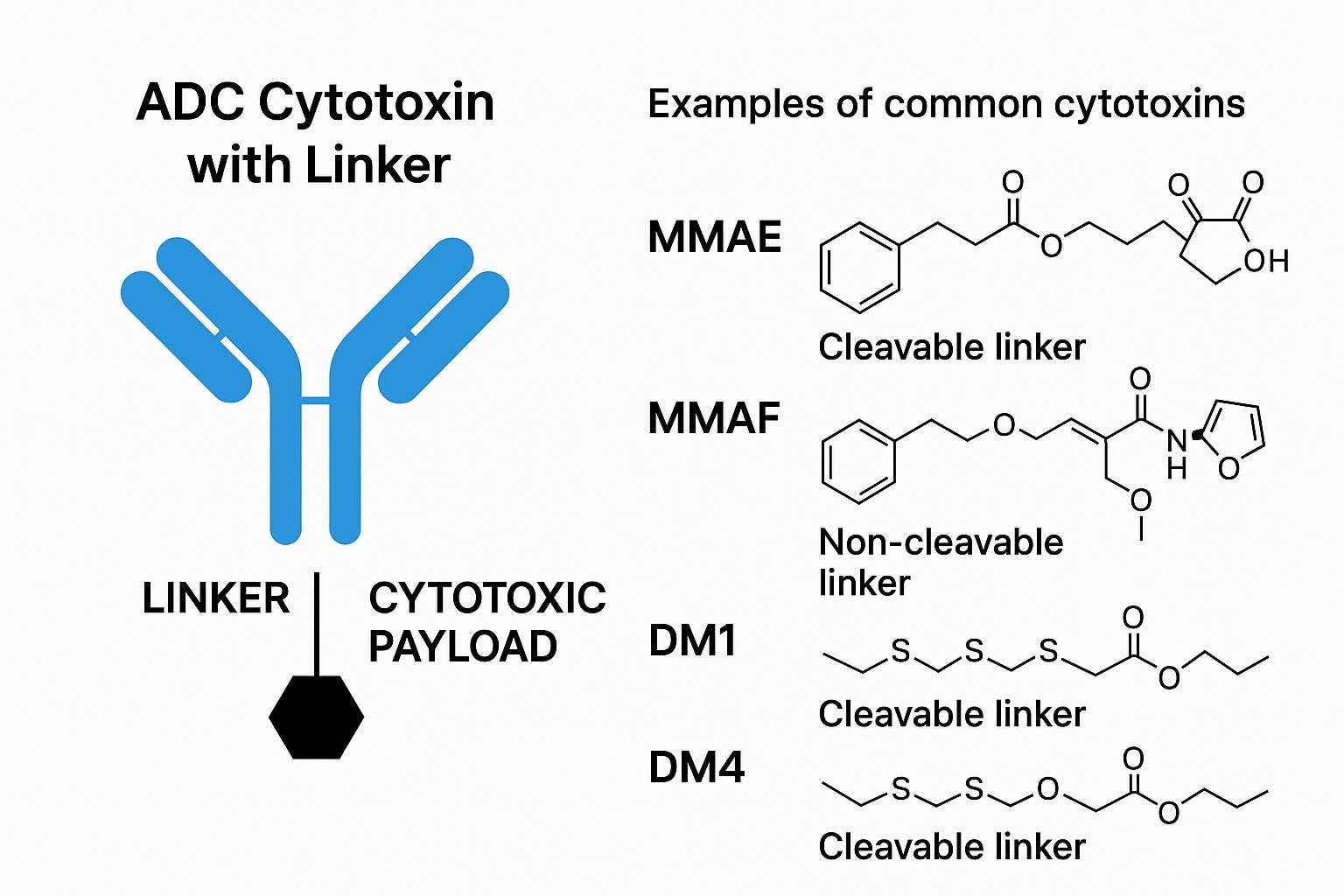
Auristatin toxins (MMAE, MMAF) are currently the most commonly used ADC payloads in several marketed products (e.g., Adcetris). BOC Sciences possesses mature Val-Cit-PABC linker conjugation technology to ensure efficient release of auristatin drugs under tumor cell lysosomal enzymes. Through advanced cysteine-based or lysine-based conjugation strategies, we provide highly homogeneous, high-DAR auristatin-ADCs, supporting the full process from R&D to preclinical stages.
Pyrrolobenzodiazepine (PBD) dimers are ultra-potent DNA crosslinkers capable of inducing tumor cell apoptosis at very low doses. BOC Sciences specializes in developing self-immolative linkers to achieve stable attachment of PBD to antibodies, minimizing the nonspecific toxicity risk caused by free toxins. We provide a highly safe operational environment and strict hazardous substance management systems to ensure the feasibility and safety of PBD conjugation processes.
SN-38, the active metabolite of irinotecan, has broad-spectrum antitumor activity but faces challenges in conjugation development due to poor water solubility and low stability. BOC Sciences offers innovative PEG-modified linkers and cleavable linker designs to effectively improve the solubility and in vivo stability of SN-38 conjugates. We support high DAR design, scale-up production, and comprehensive analytical validation to help clients accelerate SN-38-ADC development.
BOC Sciences fully understands the complexity and high requirements of ADC development. Therefore, we have designed a service system covering the entire ADC conjugation lifecycle. From innovative linker design, precise cytotoxin synthesis, to final antibody-drug conjugation and rigorous quality characterization, we are committed to providing efficient and reliable one-stop solutions for global research institutions and pharmaceutical companies.
ADCs linkers can significantly impact the toxicity, stability, specificity, and other properties of ADC drugs. Therefore, the choice of linkers is critical for ADC drug development. BOC Sciences' linker development platform can provide various linker products and development services for your research and production needs, including cleavable and non-cleavable linkers.
Multiple Types of Linkers Customized
Linker Structure Optimization
The cytotoxic payload usually utilizes small-molecule drugs targeting tumor cells/tissues to induce cell death. Cytotoxins are the killing core of ADCs, and their selection and handling must be strictly controlled. BOC Sciences offers synthesis and modification services for various efficient cytotoxins and their derivatives, including:
Classic Cytotoxins
Efficient Conjugation Strategies
The connection between payload and antibody is crucial for regulating ADC consistency and efficacy. Traditional methods link cytotoxic molecules to antibodies via lysine and interchain cysteine residues. However, new site-specific conjugation technologies enable generation of ADCs with single DAR values using unique linkers. Efficient conjugation of antibody and linker-cytotoxin complexes is the core step in ADC production.
Precise Conjugation
DAR Control and Comprehensive Quality Characterization
To meet clients' needs from early research to preclinical and clinical stages, BOC Sciences offers process optimization and scale-up production services. We deliver linker-toxin custom services throughout drug discovery in the most cost-effective, controllable, and predictable manner. Our advanced scientific team promptly provides targeted linker-toxin process development support.
Process Development
Scale-Up Production Capacity
We provide comprehensive services covering the entire process from linker and cytotoxin synthesis to conjugation, characterization, and process optimization, helping clients efficiently advance ADC projects while saving time and costs.
Expertise in bioorthogonal chemistry and highly selective conjugation strategies effectively reduces the risk of nonspecific modifications, ensuring the homogeneity and stability of ADC products.
Equipped with highly sensitive analytical instruments to monitor DAR, purity, and structural integrity in real time, meeting international quality standards such as ICH Q6B.
Tailored design of linker and cytotoxin structures based on client needs, supporting full-cycle technical services from proof-of-concept to preclinical materials.
Facilities compliant with ISO and cGMP standards and strict biosafety management ensure the safe production of highly active raw materials and conjugated products.
Our technical team consists of experienced chemists and ADC experts with successful development experience in large international ADC projects.
Capable of meeting varying demands from milligram-scale R&D to kilogram-scale production, flexibly accommodating clients' project progress at different stages.
Continuously optimizing linker and cytotoxin structures, exploring novel conjugation technologies to enhance the therapeutic window and market competitiveness of ADC drugs.

Our expert team communicates closely with clients to comprehensively evaluate project requirements, including antibody type, payload selection, linker design, and target DAR. We tailor personalized solutions based on the client's R&D phase and provide detailed project plans and timelines.
According to project demands, BOC Sciences conducts custom synthesis of linkers and cytotoxins using advanced organic synthesis and purification technologies, ensuring delivery of high-purity, highly stable linkers and cytotoxins that meet subsequent conjugation process development requirements.
Through experimental validation and process adjustments, we develop and optimize conjugation strategies for antibodies and linker-cytotoxin complexes, focusing on controlling the DAR, reducing nonspecific modifications, and ensuring conjugate homogeneity and stability, culminating in a comprehensive process report.
Comprehensive analysis of conjugates including structural confirmation, DAR determination, purity testing, and long-term stability evaluation. Employing multiple advanced techniques (LC-MS, HPLC, SEC-MALS, etc.), we provide complete analytical data packages compliant with international standards.
Upon process validation, we support scale-up from laboratory to production scale, ensuring batch consistency and high yields. Production strictly follows cGMP standards, accompanied by comprehensive quality verification and detailed scale-up and batch production reports.

High-quality ADC conjugates are delivered to clients on schedule, along with technical support and follow-up optimization suggestions to assist smooth progress in preclinical and clinical research. BOC Sciences also offers ongoing collaboration and technology upgrades according to client needs.
BOC Sciences offers a comprehensive range of linkers and cytotoxins for ADC projects. Linker types include cleavable (e.g., Val-Cit, disulfide, acid-sensitive linkers) and non-cleavable (e.g., PEG derivatives, maleimide) structures to accommodate various release strategies. Cytotoxins include highly active compounds such as MMAE, MMAF, PBD dimers, Calicheamicin, and SN-38, supporting diverse payload requirements. We can also develop novel structures tailored to special project needs to ensure optimal compatibility and efficacy.
Yes. Our team specializes in tailoring linker structures to client specifications, fully considering antibody type, payload characteristics, and target cell microenvironment. We optimize structure to balance hydrophilicity/hydrophobicity, reactivity, and biocleavability, ensuring linker stability and precise release both in vivo and in vitro to maximize ADC efficacy. Our full-cycle customization services—from design and synthesis to validation—comprehensively support your R&D plans.
We utilize multiple advanced conjugation strategies, including cysteine-based, lysine-based, and site-specific conjugation, combined with process optimization for precise DAR control. By real-time monitoring and reaction condition optimization, we ensure uniform drug loading (DAR), enhancing ADC stability and therapeutic window while reducing aggregation or nonspecific toxicity risks.
BOC Sciences is equipped with advanced analytical platforms including LC-MS, HPLC, SEC-MALS, and more, enabling comprehensive analysis of ADC purity, structural integrity, DAR, and stability. We employ methods compliant with international quality standards such as ICH Q6B and deliver full analytical reports supporting clinical submissions and quality audits, helping clients validate product quality and consistency.
We have flexible production capacity capable of delivering from milligram-scale laboratory samples to gram-scale preclinical materials and kilogram-scale manufacturing. Whether your project is in early R&D, preclinical, or clinical stages, we respond promptly to ensure efficient production, timely delivery, and strict adherence to cGMP standards and batch consistency requirements.
Yes. BOC Sciences' manufacturing facilities comply with ISO and cGMP regulations, enabling synthesis and production of highly active raw materials and ADC conjugates under high safety standards. We provide cGMP-grade linker and cytotoxin materials with complete production records and quality control systems to support client needs throughout preclinical and clinical phases.
Yes. We have dedicated high-activity drug management systems and secure operation platforms to safely and effectively handle highly toxic cytotoxins such as PBD dimers and Calicheamicin. Our team is rigorously trained for handling hazardous compounds and performs all operations in biosafety level-compliant environments, ensuring production safety and product quality.
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










