BOC Sciences possesses GMP and ISO-certified contract manufacturing facilities, and we provide custom cGMP-grade antibody-drug conjugates (ADC) manufacturing services to meet clinical and commercial development needs. We offer end-to-end ADC manufacturing services covering laboratory-scale studies, process development, pilot-scale production, and GMP manufacturing. Supported by an advanced conjugation technology platform, a robust quality management system, and an experienced technical team, our strong expertise in process development, characterization, optimization, and scale-up production ensures successful GMP manufacturing. We have established productive long-term collaborations with pharmaceutical companies, institutions, and universities.
ADCs are formed by conjugating monoclonal antibodies (mAb) and small-molecule drugs through linkers. The specificity of mAb to tumor cells allows small-molecule drugs to target tumor tissues, effectively reducing the high toxicity of traditional small-molecule drugs and improving overall treatment efficiency. Most ADCs follow similar action mechanisms, including antibody-mediated receptor binding, ADC internalization, the release of payload cytotoxicity, and bystander effects. The success of ADC depends on several key factors: target antigens, antibody types, payload types, linker types, conjugation methods, and target indications.
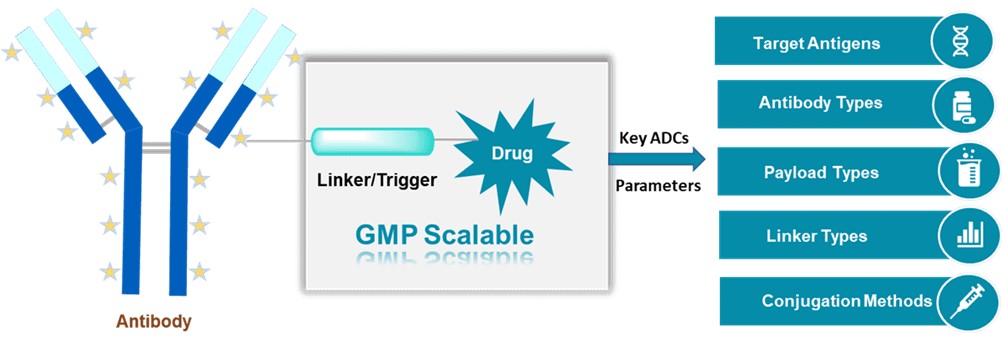 Fig 1. GMP capability for payloads and linkers (BOC Sciences Authorized).
Fig 1. GMP capability for payloads and linkers (BOC Sciences Authorized).
BOC Sciences offers comprehensive services for ADC manufacturing, including antibody production, linker chemistry, payload conjugation, and formulation development. We have a team of experienced scientists and engineers who are well versed in various technologies and have expertise in ADC development. We also provide custom solutions and can customize specific ADC manufacturing processes to meet specific customer requirements. We have a strong focus on collaboration and work closely with our clients to understand their needs and provide a personalized services.
The advent of ADCs provides a promising treatment for many types of cancer. As increasing numbers of ADC drugs enter clinical trials, the industry is shifting gradually from traditional technologies to novel and powerful techniques to afford ADCs. These techniques include exploring new tumor antigens, new antibody structures, new payloads, new linkers, and advanced conjugation methods that improve the therapeutic window of ADC. BOC Sciences offers a complete and flexible range of ADC manufacturing services, from process development & analytical support to GMP production. Our services include biopharmaceutical contract research and development, process development, cGMP manufacturing, QA and QC solutions for scientific research, preclinical, clinical, and commercial supply.
We have ADC manufacturing facilities and systems compliant with international cGMP standards, supporting production and delivery from preclinical samples to commercial batches. The manufacturing process covers the entire workflow including antibody reduction, linker and cytotoxin conjugation, purification, concentration, and sterile fill-finish, ensuring each step meets quality and regulatory requirements.
Service Highlights:
Efficient purification is a critical step to ensure the quality and safety of ADC products. We employ multiple purification technologies including hydrophobic interaction chromatography (HIC), ion exchange chromatography (IEC), size exclusion chromatography (SEC), and tangential flow filtration (TFF) to remove unconjugated toxins, free antibodies, and other impurities.
Service Highlights:
We provide professional ADC formulation development services to help clients optimize product stability, reduce aggregation issues, and improve shelf life. Formulation strategies are tailored based on the physicochemical properties of the ADC, intended administration routes, and clinical requirements, selecting the most suitable buffer systems and excipient combinations.
Service Highlights:
We offer GMP-compliant sterile fill-finish services for ADCs, including both liquid and lyophilized powder forms, ensuring product stability, safety, and traceability throughout formulation and filling. The entire process is conducted in Grade A/B cleanrooms equipped with high-precision filling equipment and comprehensive aseptic control systems.
Service Highlights:


| AD Systems and Equipment | QC Lab Systems and Equipment | |
| HPLC 2DHPLC | Instrument Group | HCP |
| Bio-LC | SEC-HPLC | Residual Host Cell DNA |
| cIEF (iCE3) | cIEF | Residual Protein A |
| CE (PA800) | Peptide Mapping (UPLC) | Protein Concentration by UV |
| Q-TOF ( Agilent 6530B) | IEX-HPLC (Ion-Exchange) | EM/Microbiology Group |
| UPLC ( Agilent 1290 Infinity) | CE-SDS (reduced and non-reduced) | Bioburden |
| CE (Agilent) | Biochem/Chem Group | Endotoxin |
| Flow Cytometry | Cell-based Bioassay | Sterility |
| GC | ELISA / Western Blot | Water Testing |
| qPCR instruments | Trypsin Inhibitory Activity | Environmental Monitoring |
| 2DHPLC | SDS-PAGE (reduced and non-reduced) | Particulate Matter |

At the project initiation stage, we closely communicate with clients to understand the ADC components (antibody, linker, cytotoxin) and project goals, assess process feasibility, and complete the necessary technology transfer tasks.
For the ADC structures provided by clients, we conduct process development and parameter optimization to ensure a stable and controllable scale-up from small scale to pilot scale, compatible with subsequent GMP manufacturing.
Using multiple chromatography techniques and filtration methods, we efficiently purify the ADC reaction mixtures to remove free toxin, unconjugated antibody, and other impurities, ensuring the purity and safety of the final product.
To enhance the storage stability and formulation performance of ADC products, we develop and screen suitable formulation systems and determine final formulation parameters.
Following purification and formulation development, the ADC undergoes final fill-finish under GMP-compliant sterile conditions to ensure compliance with clinical use requirements.

Quality control is integrated throughout the entire ADC manufacturing process. We have an independent QC laboratory conducting comprehensive quality testing and release review of raw materials, intermediates, and final products.
ADC contract manufacturing provides end-to-end support for developing and producing antibody drug conjugates, offering specialized facilities, process development, and regulatory expertise to accelerate time to market.
The ADC manufacturing process includes antibody production, drug-linker synthesis, conjugation, purification (e.g., TFF), sterile fill-finish, and quality control, all performed under strict contamination control.
TFF is essential for purifying ADCs, removing unbound drug-linkers and contaminants, concentrating the final product, and ensuring high-quality and safe drug formulations for clinical and commercial use.
Large-scale ADC production must manage high potency materials, prevent contamination risk, maintain batch consistency, and meet GMP standards, especially during process scale-up and supply chain integration.
Specialized ADC manufacturing facilities use containment systems, single-use technologies, validated cleaning protocols, and quality management systems to minimize contamination risk and ensure high-quality outputs.
We offer one-stop ADC manufacturing services covering the entire process from process development and GMP-grade production to fill and finish. Our capabilities include antibody production, linker and payload synthesis, conjugation, purification, tangential flow filtration (TFF), and quality control.
Yes, our manufacturing facilities are cGMP-compliant and support both clinical-stage and commercial ADC production, ensuring product quality, batch consistency, and regulatory compliance.
We offer flexible production capabilities to meet different project needs, from milligram to kilogram-scale ADC formulation manufacturing, supporting early R&D, pilot-scale production, and commercial supply.
From cytotoxin synthesis to linker design, discover our specialized services that complement your ADC projects.
Find exactly what your project needs from our expanded range of ADCs, offering flexible options to fit your timelines and goals.
Contact our experts today for pricing and comprehensive details on our ADC offerings.










