Antibody-drug conjugates (ADCs), as an important form of precision targeted tumor therapy, owe their clinical success not only to the specificity of the antibody but are also largely limited by the solubility of the payload and the overall stability of the conjugate. With the continuous evolution of ADC technology, researchers and companies are actively exploring a range of innovative strategies to overcome challenges such as poor solubility, aggregation, and in vivo instability.
ADCs combine the targeting ability of monoclonal antibodies with the potent cytotoxicity of small molecule drugs, making them a focus of recent anti-cancer drug development. However, during ADC development and application, solubility and stability remain key factors restricting their clinical success. The following will deeply explore the core elements affecting ADC solubility and stability.
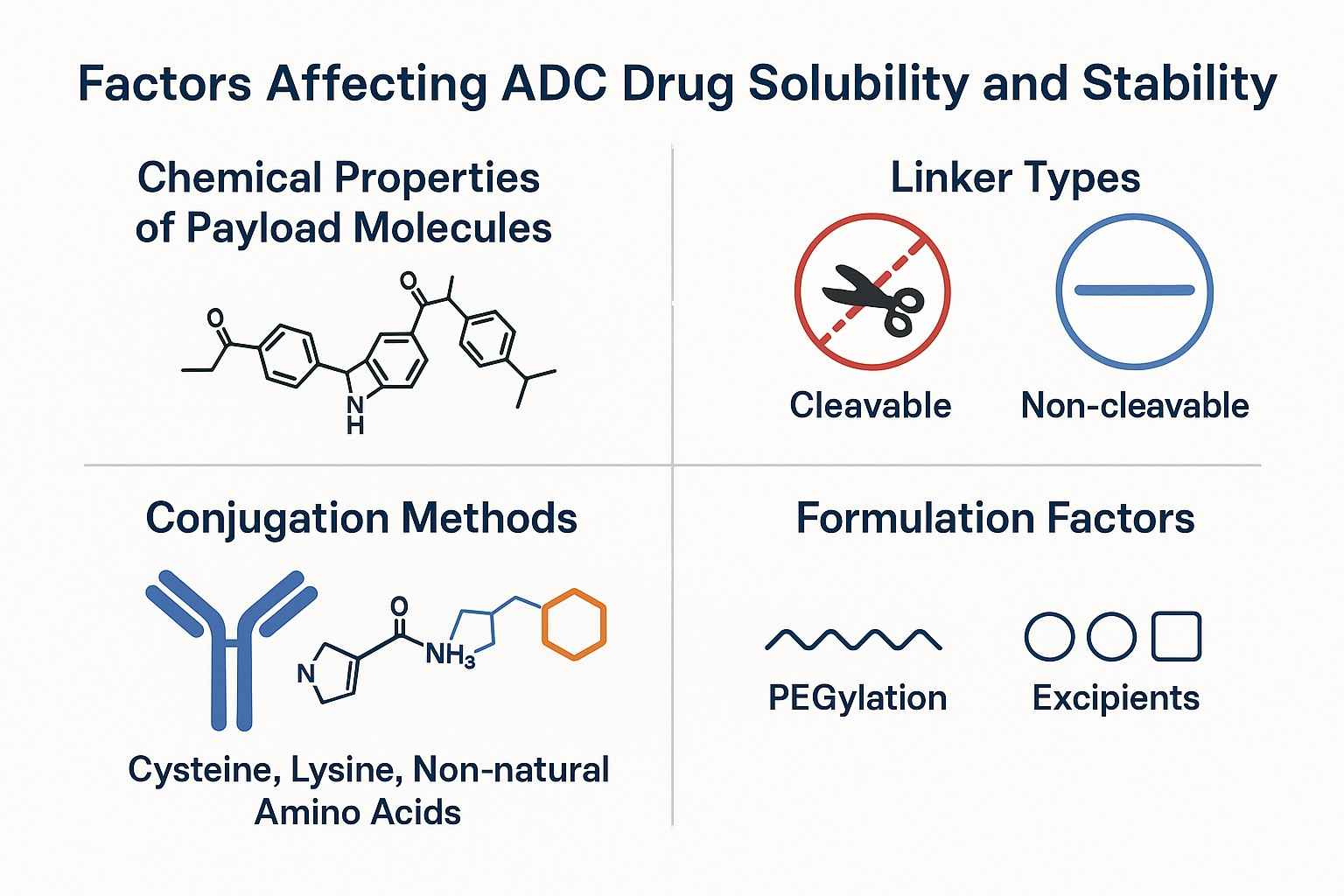 Fig. 1. Elements impacting ADC drug solubility and stability (BOC Sciences Authorized).
Fig. 1. Elements impacting ADC drug solubility and stability (BOC Sciences Authorized).
The chemical characteristics of payload molecules are the core factors influencing ADC solubility and stability. Most cytotoxic drugs used in ADCs are highly hydrophobic. While this property enhances intracellular penetration, it also leads to non-specific aggregation of ADC molecules in plasma, thereby affecting solubility and bioavailability. The molecular weight, spatial structure, and surface charge distribution of the payload molecule also significantly alter the physicochemical properties of ADCs. For example, when the drug-antibody ratio (DAR) is too high, hydrophobicity increases, aggregation tendency rises, which may trigger immunogenic responses and pharmacokinetic abnormalities. Therefore, in ADC design, the chemical properties of the payload play a decisive role in overall stability and therapeutic efficacy.
The chemical structure of the linker directly relates to ADC stability in vivo and the precision of drug release. The linker not only serves as a bridge connecting the drug to the antibody but also determines when and where the drug is released. Cleavable linkers (such as disulfide bonds and ester bonds) can effectively release drugs inside target cells but often exhibit poor stability in the bloodstream, leading to premature payload release that compromises ADC targeting and safety. In contrast, non-cleavable linkers (such as thioether bonds and hydrazone bonds) provide higher plasma stability but may limit the efficiency of active drug release within target tissues. Moreover, hydrophilic or hydrophobic modifications in linker structure significantly impact the overall solubility and aggregation behavior of ADC molecules.
Conjugation sites and methods are important factors determining ADC product homogeneity and stability. Traditional lysine and cysteine conjugation methods, due to random binding positions, often lead to heterogeneity in ADC molecules, manifested as uneven DAR, causing solubility differences and unstable pharmacokinetic profiles. Next-generation site-specific conjugation technologies achieve precise payload attachment at specific antibody sites by engineering antibodies or incorporating non-natural amino acids, greatly enhancing product uniformity and stability. This precise conjugation not only improves ADC distribution and efficacy in vivo but also reduces immunogenic risks caused by aggregation, making the products more suitable for clinical application.
The complex structure and multi-component nature of ADCs make stability evaluation a key aspect of research and quality control. To ensure the safety and efficacy of ADCs during development, storage, and clinical application, the development and application of highly sensitive analytical tools are crucial. These tools can detect aggregation, degradation, and drug release of ADCs under various conditions, as well as monitor changes in DAR in real time, providing data support for formulation optimization and manufacturing process control.
Aggregation and degradation of ADCs are critical indicators for assessing their quality and clinical safety. Aggregation may increase immunogenicity and cause pharmacokinetic abnormalities, while degradation affects ADC efficacy and stability. Common detection techniques include size exclusion chromatography (SEC), dynamic light scattering (DLS), and transmission electron microscopy (TEM), which accurately analyze ADC aggregation states and particle size distribution under different environments. Additionally, high-performance liquid chromatography (HPLC) and mass spectrometry (MS) are often used to monitor chemical degradation products, such as linker cleavage or payload loss. Using these advanced analytical tools, researchers can monitor ADC stability during storage, transport, and in vivo conditions, guiding formulation and process improvements.
DAR is a key parameter measuring ADC consistency and efficacy. Fluctuations in DAR directly impact ADC potency, solubility, and in vivo distribution, so precise determination of its stability is essential. Common techniques include liquid chromatography-mass spectrometry (LC-MS), capillary electrophoresis (CE), and ultraviolet (UV) spectroscopy, which quantitatively analyze DAR changes under various conditions and detect premature payload detachment or incomplete release. High-resolution mass spectrometry is especially suitable for detecting subtle differences in complex samples, helping to ensure consistent ADC quality throughout the development cycle.
Real-time monitoring of ADC stability in simulated or actual physiological environments is an important step in evaluating clinical suitability. Emerging technologies such as biolayer interferometry (BLI), surface plasmon resonance (SPR), and microfluidic chips can dynamically detect ADC behavior in complex environments like plasma and tissue fluids, including antibody integrity, linker stability, and drug release rate. The application of these techniques allows researchers to more accurately predict in vivo ADC performance and identify potential stability issues early. Additionally, real-time monitoring supports long-term stability assessments under storage conditions, providing crucial data for formulation development and quality control of ADCs.
In the design of ADCs, the mode of payload release directly determines therapeutic efficacy and side effect control. An ideal ADC should remain stable in circulation to avoid premature drug release, while achieving efficient drug release inside target cells. To achieve this goal, researchers continuously optimize ADC solubility and controlled drug release through payload structural modifications, application of hydrophilic linkers, and nanocarrier technologies. These strategies not only improve ADC pharmacokinetic properties but also significantly enhance the precision of tumor-targeted therapy.
Structural adjustment of payload molecules is an important approach to improve ADC solubility. Many cytotoxic drugs are inherently highly hydrophobic, which easily causes ADC aggregation and plasma instability. By introducing hydrophilic substituents or making mild chemical modifications, payload water solubility can be improved while retaining anti-tumor activity. Such molecular-level adjustments help ADC distribute evenly in the bloodstream, reduce aggregation and precipitation risks, and enhance formulation process feasibility.
Hydrophilic linkers and polyethylene glycol (PEG) modification techniques are widely used to enhance ADC solubility and in vivo stability. Linkers containing hydrophilic segments, such as PEG groups, effectively reduce overall ADC hydrophobicity, decreasing non-specific binding and aggregation. Furthermore, PEGylation can provide a "stealth" protective layer for ADC molecules, prolong plasma half-life, and improve pharmacokinetic behavior. These hydrophilic modification strategies help maintain precise drug release while increasing ADC clinical safety.
Nanocarrier technology offers new ideas to address ADC payload solubility and targeted delivery issues. By encapsulating hydrophobic drugs within liposomes, polymer nanoparticles, or other nanostructures, payload water solubility and biocompatibility can be significantly improved. Such nanocarriers not only protect the payload from degradation in plasma environments but also achieve more efficient tumor targeting through surface functionalization. The combination of nanotechnology with ADCs is driving the development of next-generation targeted therapeutics, making drug delivery more precise and efficient.
| Payload Type | Description |
| Biological Payload | Utilizing biologic molecules to deliver highly specific and potent therapeutic effects in ADCs. |
| Chemical Payload | Offering a broad range of chemically optimized small molecules for effective and stable ADC payloads. |
| Nanocarrier | Employing advanced nanocarriers to enhance targeted drug delivery and improve tumor penetration efficiency. |
| Protein Toxin | Providing engineered protein toxins designed for exceptional potency and novel mechanisms in ADC therapy. |
The chemical stability of linkers directly affects the plasma half-life, targeting ability, and safety of ADCs in vivo. To overcome the problem of rapid degradation of traditional linkers in the bloodstream, researchers have developed various innovative strategies, including designing novel cleavable and non-cleavable linkers, achieving site-specific conjugation to reduce product heterogeneity, and enhancing overall ADC stability through formulation optimization. These technological advances provide critical support for improving the clinical performance of ADCs.
Cleavable and non-cleavable linkers represent two major design directions tailored to different drug release requirements. Cleavable linkers use pH-sensitive, enzyme-sensitive, or reductive environment-sensitive chemical bonds to release payloads within the tumor microenvironment or inside cells, but they may suffer from insufficient stability in plasma. Non-cleavable linkers, on the other hand, connect the payload via stable chemical bonds, with drug release occurring only after intracellular degradation of the antibody, significantly enhancing stability in circulation. Each design has its advantages, and rational selection is crucial to ensure ADC efficacy and safety.
Traditional random conjugation methods cause uneven distribution of drug loads (heterogeneity) within ADC molecules, impacting solubility, pharmacokinetics, and safety. Site-specific conjugation techniques achieve precise payload attachment by introducing defined conjugation sites on the antibody or incorporating non-natural amino acids. This precise conjugation significantly reduces batch-to-batch variability, improves product consistency and stability, and enhances in vivo distribution and targeting efficiency, meeting the higher quality control requirements of modern ADC development.
During formulation development, ADCs may face physical and chemical stability challenges such as aggregation, degradation, or payload loss. Formulation optimization effectively enhances overall ADC stability. Common approaches include adjusting buffer systems, adding stabilizers, and optimizing pH and ionic strength to maintain ADC structural integrity and functional activity. Scientific formulation design not only ensures product quality during storage and transportation but also provides more reliable stability support for clinical use.
| Linker Type | Description |
| Acid Cleavable Linkers / Hydrazone Linkers | Stable in circulation, cleaved in acidic tumor environments for payload release. |
| Disulfide Linkers | Redox-sensitive linkers cleaved by intracellular glutathione enabling controlled drug release. |
| Cathepsin B Cleavable Linkers / Peptide Linkers | Enzyme-sensitive peptide linkers selectively cleaved by Cathepsin B in tumor cells. |
| Phosphatase Cleavable Linkers | Linkers cleaved by phosphatases, facilitating selective payload release inside target cells. |
| Sulfatase Cleavable Linkers | Designed to be cleaved by sulfatases, triggering release of ADC payloads at tumor sites. |
| β-Galactosidase Cleavable Linkers | Payload release activated by β-Galactosidase enzyme overexpressed in certain tumors. |
| β-Glucuronidases Cleavable Linkers | Enzyme-responsive linkers cleaved by β-Glucuronidases for targeted intracellular payload delivery. |
ADC aggregation frequently occurs during development and storage, which not only affects drug solubility and stability but may also trigger immunogenic responses and reduce therapeutic efficacy. ADC aggregation is often caused by high payload hydrophobicity, the chemical structure of linkers, or inappropriate choice of conjugation sites. To address these issues, scientists are exploring multiple prevention strategies, including optimizing payload and linker design, introducing hydrophilic modifications, improving conjugation techniques, and adjusting formulations.
The tendency of ADCs to aggregate largely stems from the high hydrophobicity of payload molecules, which can form hydrophobic patches on the antibody surface, promoting intermolecular interactions and aggregation. By suitably modifying payload structures to reduce exposed hydrophobic regions or introducing hydrophilic segments into linker design, these hydrophobic interactions can be effectively weakened. Such molecular-level interventions help improve ADC solubility and dispersion in plasma, thereby lowering aggregation risk.
The choice of excipients plays a key role in preventing ADC aggregation. Certain surfactants, sugars, or amino acids act as stabilizers to reduce non-specific interactions between ADC molecules and maintain molecular stability in solution. For example, polysorbate surfactants are widely used in antibody formulations to prevent aggregation caused by protein interface activation. Proper excipient combinations not only improve long-term storage stability of ADCs but also enhance their safety during clinical use.
Storage and handling conditions have a significant impact on the physical stability of ADCs. Factors such as temperature fluctuations, pH changes, and mechanical stresses can induce ADC aggregation and degradation. Strict control of storage environments, avoidance of freeze-thaw cycles, and minimizing vigorous agitation are key measures for maintaining ADC quality in manufacturing and supply chain management. Additionally, developing appropriate buffer systems and stable formulations helps ensure that ADCs retain structural integrity and functional activity before and after transportation and clinical use.
Leveraging a strong chemical and biotechnological platform, BOC Sciences offers comprehensive solutions covering payload optimization, linker design, formulation development, and GMP manufacturing. Our expert team is dedicated to customizing efficient and stable ADC products for clients worldwide, accelerating your projects from research to clinical and commercial stages.
From cytotoxins to linkers, explore our cutting-edge products for your ADC project.
| Catalog | Name | CAS | Price |
| BADC-00014 | Mc-MMAF | 863971-19-1 | Inquiry |
| BADC-00372 | SPDP | 68181-17-9 | Inquiry |
| BADC-00009 | DM1-SMCC | 1228105-51-8 | Inquiry |
| BADC-00020 | DM1-SMe | 138148-68-2 | Inquiry |
| BADC-00045 | Auristatin F | 163768-50-1 | Inquiry |
| BADC-00906 | Propargyl-PEG5-acid | 1245823-51-1 | Inquiry |
| BADC-01633 | 3-Azidopropanol | 72320-38-8 | Inquiry |
| BADC-00290 | Lycorine hydrochloride | 2188-68-3 | Inquiry |
| BADC-00089 | Calicheamicin | 108212-75-5 | Inquiry |
| BADC-00780 | Spliceostatin A | 391611-36-2 | Inquiry |
Explore our advanced tools and expertise for next-generation ADC research and development.
Precise antibody engineering and conjugation to optimize ADC stability and targeting.
Comprehensive analytical services ensuring ADC quality, purity, and functional integrity.
Tailored process optimization to enhance ADC manufacturing efficiency and scalability.
Innovative formulation strategies improving ADC stability, delivery, and therapeutic performance.










