Antibody-drug conjugates (ADCs), as a significant breakthrough in tumor-targeted therapy, have therapeutic efficacy that depends not only on the selection of antibodies and toxins but is also directly influenced by the design and performance of the linker. An excellent linker design can ensure the stability of the ADC in vivo and enable precise release at tumor cells, thereby improving therapeutic efficiency and reducing off-target toxicity. This article will systematically explore the critical role of ADC linkers, challenges in development, application scenarios of different types of linkers, optimization strategies, and comprehensive support provided by BOC Sciences in ADC linker development.
As the critical bridge between antibody and cytotoxin, the ADC linker directly affects the drug's targeting ability, stability, and toxin release efficiency. It not only ensures the drug remains stable in the bloodstream to prevent premature release but also cleaves precisely under specific conditions within tumor cells to achieve effective drug release. Optimizing linker design is a core step to enhance the efficacy and safety of ADCs.
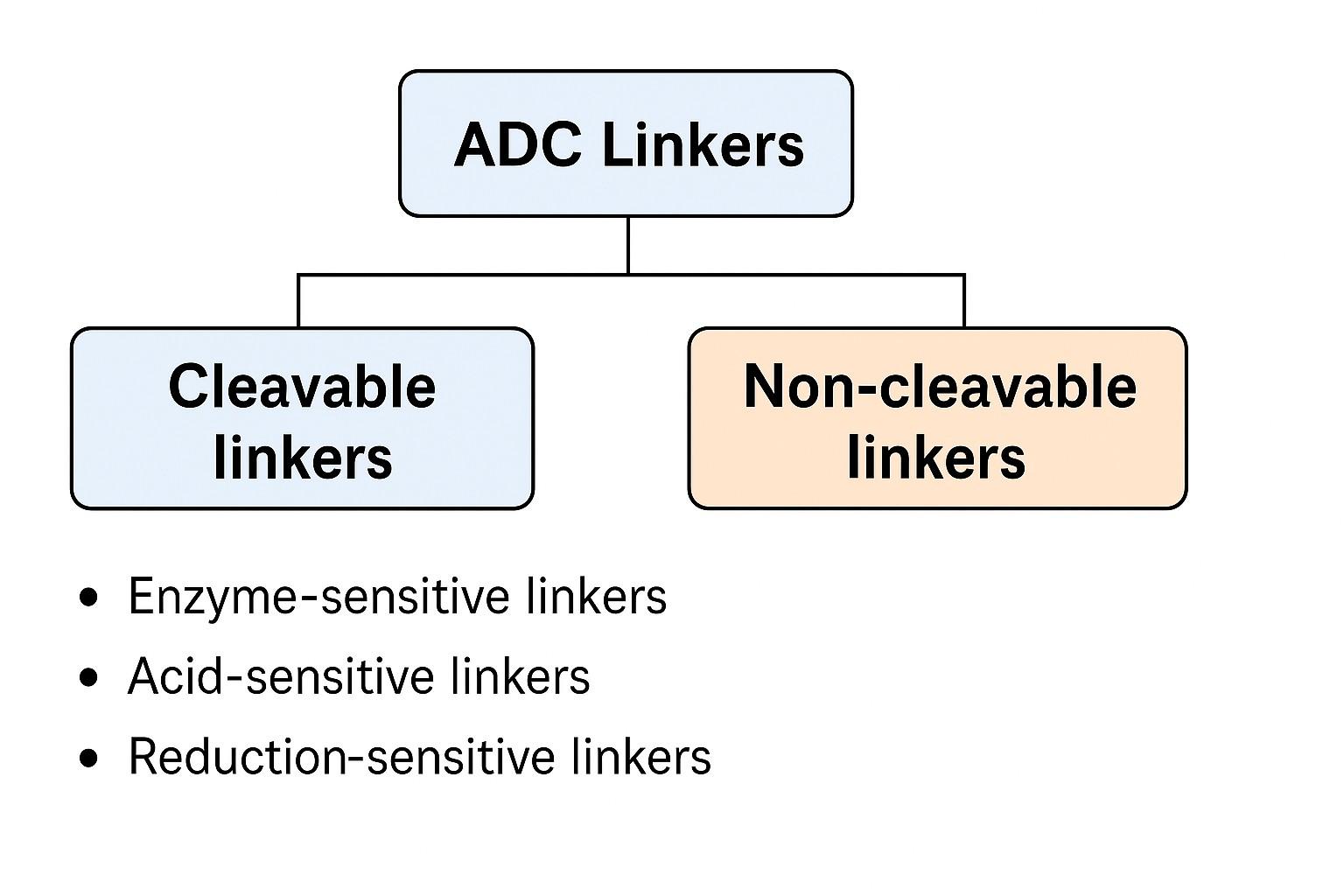 Fig. 1. Current adc linker chemistry (BOC Sciences Authorized).
Fig. 1. Current adc linker chemistry (BOC Sciences Authorized).
In the complex therapeutic system of ADCs, the linker is the chemical bridge connecting the antibody and the small-molecule cytotoxin, bearing the dual responsibilities of stable transport and selective release. Although its structural proportion within the whole ADC molecule is relatively small, its function and performance directly determine whether the ADC can exert the desired therapeutic effect in patients. An ideal ADC linker should meet the following key requirements:
Therefore, linker design is not only a chemical engineering challenge but also a key node in precision medicine. An optimized linker can precisely control the ADC's behavior in vivo, including pharmacokinetics, tissue distribution, toxin release rate, and ultimately therapeutic outcome. In short, the linker is the hub connecting the "design intent" and the "clinical performance" of the ADC; its quality will directly affect the therapeutic window, adverse effects, efficacy consistency, and patient safety.
The role of the linker in drug targeting is twofold. First, it must maintain ADC stability during circulation to prevent premature drug release before reaching the target site. This stability is essential to ensure that sufficient ADC reaches the intended destination. Second, the linker must facilitate drug release after ADC internalization by target cancer cells. This controlled release mechanism is critical to maximizing the cytotoxic effect on cancer cells while minimizing exposure to healthy tissues. The release mechanism is influenced by the linker's sensitivity to intracellular conditions such as pH, enzymes, or other biochemical environments.
Although ADC technology has made significant progress in precise cancer therapy, many complex challenges remain in its development, especially regarding linker design and application. The chemical structure, reaction mechanism, synthetic process, and compatibility issues of linkers may all affect the ADC's efficacy, safety, and manufacturability.
One major challenge in ADC linker development is achieving optimal stability. The linker must be stable enough to prevent premature drug release during circulation but labile enough to cleave once inside the target cell to release the drug. Achieving this balance is complex because an overly stable linker may prevent drug release, whereas an overly labile linker may cause premature release and off-target toxicity. Ensuring stability also requires considering the linker's resistance to proteolysis, hydrolysis, or other potential degradation pathways in the bloodstream.
Premature drug release is a critical issue in ADC development. If the drug detaches from the antibody before reaching the target site, it can cause systemic toxicity and reduce therapeutic efficacy. This problem is especially challenging because the linker must remain stable under the diverse and harsh conditions of the bloodstream, including exposure to enzymes, varying pH values, and other biological factors. Overcoming premature release requires meticulous linker design to ensure its integrity until the ADC arrives at the target tissue.
Another challenge is balancing linker strength with drug efficacy. A linker that is too robust may block drug release, while one that is too fragile may lead to premature drug release. Additionally, linker design must consider the drug's mechanism of action and the characteristics of the target cells. For example, if the drug requires a specific intracellular environment to be activated, the linker must be designed to release the drug under those conditions. Balancing these factors requires in-depth understanding of both the drug and target biology.
In ADC construction, selecting the linker type is a key factor that determines the drug release pathway, safety, and therapeutic efficacy. According to cleavage mechanism and release mode, ADC linkers are generally divided into two categories: cleavable linkers and non-cleavable linkers. Both have advantages and suit different targeting strategies and clinical needs. A deep understanding of their structural characteristics and compatibility conditions during linker development helps achieve more precise ADC pharmacological control.
Cleavable linkers are designed to break under specific physiological or pathological conditions to release the toxin. They typically exploit biochemical differences between the tumor microenvironment and normal tissues (such as pH, enzyme expression, or reduction potential) to achieve precise release. Common types include:
These linkers are suitable for ADCs that require rapid toxin release within cells or the tumor microenvironment and are widely used in tumors with heterogeneous target expression and needing fast cytotoxic action.
Non-cleavable linkers require the ADC to enter the cell and release the toxin via complete antibody degradation pathways. These linkers exhibit high stability and generally do not cleave spontaneously in the blood or tumor microenvironment. The released toxin is usually in a conjugated form with antibody residues. Typical examples include SMCC linkers (succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate), often used to conjugate with microtubule inhibitors (like MMAF). Non-cleavable linkers are suitable for targets with high internalization efficiency and clear intracellular degradation pathways. They offer better pharmacokinetic stability and controlled release, reduce systemic toxicity, prolong plasma half-life, and are core choices for many next-generation ADC products.
The cleavage mechanism of the linker is closely related to the tissue characteristics of the target. For example:
Additionally, compatibility of the linker with the ADC construction process must be considered. Linkers should avoid non-specific side reactions with functional groups on the antibody or toxin to ensure conjugation efficiency, product consistency, and toxin activity.
The physicochemical properties of the linker not only determine its cleavage mechanism but also affect the ADC's overall pharmacokinetic (PK) and pharmacodynamic (PD) behaviors. Key factors include:
Therefore, linker design during ADC development should not only focus on the release mechanism but also comprehensively consider its performance throughout drug construction, in vivo transport, degradation, and metabolism. A rationally structured, functionally matched linker is key to achieving therapeutic precision and broad adaptability of ADCs.
| Linker Type | Description |
| Acid Cleavable Linkers / Hydrazone Linkers | We develop acid-sensitive hydrazone linkers that enable controlled drug release in acidic tumor microenvironments or endosomal compartments. |
| Disulfide Linkers | Our disulfide linkers are engineered for reductive cleavage under high intracellular glutathione levels, ensuring tumor-selective payload release. |
| Cathepsin B Cleavable Linkers / Peptide Linkers | We offer custom peptide linkers, such as Val-Cit, specifically designed for cleavage by Cathepsin B overexpressed in tumor cells. |
| Phosphatase Cleavable Linkers | We design phosphatase-responsive linkers that exploit elevated phosphatase activity in tumors for selective and efficient drug release. |
| Sulfatase Cleavable Linkers | Our sulfatase-cleavable linkers utilize tumor-associated sulfatase enzymes to achieve targeted and enzyme-triggered drug delivery. |
| β-Galactosidase Cleavable Linkers | We develop β-galactosidase-sensitive linkers for precise drug release in cancer cells with high β-galactosidase activity. |
| β-Glucuronidases Cleavable Linkers | Our β-glucuronidase-cleavable linkers are tailored for tumor-selective activation based on the elevated β-glucuronidase levels in the tumor microenvironment. |
The structure of the linker not only determines the stability and targeted release performance of the ADC but also directly affects its pharmacokinetics, tissue distribution, and toxicity levels in vivo. Therefore, optimizing linker design has become one of the key strategies to improve ADC therapeutic efficacy and development success rate. With the advancement of molecular engineering, tumor microenvironment research, and chemical synthesis technologies, researchers can systematically improve linker performance from three aspects: stability regulation, release mechanism control, and targeting specificity, thereby endowing ADCs with a higher therapeutic window, lower systemic toxicity, and better clinical adaptability. The following will explore these three major strategies: improving linker stability, achieving controlled release, and enhancing target specificity.
ADC drugs face many challenges in blood circulation, among which the stability of the linker is a key factor to ensure that the drug does not undergo nonspecific release before reaching the target. An unstable linker can cause premature release of the cytotoxin in the system, leading to toxic side effects and reduced treatment selectivity. Therefore, improving the chemical stability of the linker has become an important direction in optimizing ADC structural design. Strategies to improve linker stability include:
Besides stability, the "controlled cleavage" ability of the linker is also a core requirement for achieving efficient ADC therapy. Engineering linker design aims to regulate toxin release at specific times, locations, and conditions. By adjusting the linker's sensitivity mechanisms (such as the length of specific enzyme recognition sequences, pKa regulation, etc.), more precise control of drug release timing and rate can be realized. For example:
Traditional linkers mainly rely on broadly present intracellular enzymes or physical environmental features (such as acidity) for cleavage. With a deeper understanding of tumor microenvironment heterogeneity, researchers have begun exploring more tumor-selective linker designs. In recent years, attention has turned to the development of "tumor-specific responsive" linkers. Such linkers activate cleavage only under certain tumor conditions (such as high GSH concentration or specific protein expression). For example:
These novel linkers provide broader possibilities for personalized ADC drug design.
As a leading life science service provider, BOC Sciences has long been engaged in ADC drug development and accumulated rich experience in linker design and synthesis. In the ADC R&D process, linker innovation and optimization often determine the final drug efficacy and safety. For different client project needs, BOC Sciences offers one-stop solutions from linker molecular design, synthetic process development to pilot scale-up and quality control. By integrating advanced chemical synthesis technologies with precise biological analysis platforms, BOC Sciences not only helps clients accelerate linker development cycles but also ensures products possess high stability, high purity, and consistency.
From cytotoxins to linkers, explore our cutting-edge products for your ADC project.
| Catalog | Name | CAS | Price |
| BADC-00008 | Val-Cit-PAB-MMAE | 644981-35-1 | Inquiry |
| BADC-00016 | Vat-Cit-PAB-MMAD | 1415329-13-3 | Inquiry |
| BADC-00029 | MC-Val-Cit-PAB-MMAE | 646502-53-6 | Inquiry |
| BADC-00369 | Fmoc-Val-Cit-PAB-PNP | 863971-53-3 | Inquiry |
| BADC-00405 | NHS-PEG4-azide | 944251-24-5 | Inquiry |
| BADC-00498 | Propargyl-NHS Ester | 1174157-65-3 | Inquiry |
| BADC-00610 | Mc-Val-Cit-PAB-Cl | 1639351-92-0 | Inquiry |
| BADC-00942 | Fmoc-Val-Ala-PAB-OH | 1394238-91-5 | Inquiry |
| BADC-00972 | Fmoc-Gly-Gly-Phe-OH | 160036-44-2 | Inquiry |
| BADC-01482 | Fmoc-Val-Ala-OH | 150114-97-9 | Inquiry |
Explore our advanced tools and expertise for next-generation ADC research and development.
Designed for tumor-triggered payload release with precise control.
Enable stable payload retention for enhanced intracellular activity.
Responsive to pH or redox for targeted drug release.
Activated by tumor-specific enzymes for selective release.










