Boc-L-Aza-OH CHA is a click chemistry reagent containing an azide group.

| Catalog Number | Size | Price | Quantity | |
|---|---|---|---|---|
| BADC-01825 | -- | $-- | Inquiry |
Boc-L-Aza-OH (CHA) stands out as a highly versatile compound utilized in peptide synthesis and diverse chemical biology applications. Below are four key applications of Boc-L-Aza-OH (CHA):
Peptide Synthesis: Central to peptide synthesis, Boc-L-Aza-OH (CHA) serves as a cornerstone for constructing peptides and small proteins. Its steadfast Boc-protecting group enables sequential coupling reactions, facilitating the assembly of intricate peptide sequences. This reagent plays a critical role in generating therapeutic peptides and research tools, vital for peptide-based investigations.
Drug Development: In the realm of drug discovery, Boc-L-Aza-OH (CHA) emerges as a pivotal component for crafting peptide-based inhibitors or mimetics. By integrating this compound into peptide structures, researchers can engineer molecules tailored to target specific protein-protein interactions or enzyme active sites, offering a path to developing highly specific and potent new drugs.
Chemical Biology: Within the domain of chemical biology, Boc-L-Aza-OH (CHA) proves invaluable for delving into protein function and interaction mechanisms. Through the integration of this compound into peptides, scientists can devise probes capable of binding to select proteins or modulating their activities. This approach aids in unraveling intricate protein networks and discerning the individual roles of proteins in cellular processes.
Structural Biology: Exploiting the attributes of Boc-L-Aza-OH (CHA) in structural biology enables the elucidation of peptide and protein structures. Its role in stabilizing peptide conformations facilitates the crystallization or NMR studies essential for understanding three-dimensional protein structures. This comprehension is crucial for deciphering functional mechanisms and designing therapeutics guided by structural insights.

Customer Support
Providing excellent 24/7 customer service and support

Project Management
Offering 100% high-quality services at all stages

Quality Assurance
Ensuring the quality and reliability of products or services

Global Delivery
Ensuring timely delivery of products worldwide

BOC Sciences offers comprehensive services for ADC manufacturing, including antibody modification, linker chemistry, payload conjugation, and formulation development. In particular, our payload-linker customization service offers a convenient and fast raw material channel for many ADC researchers.
BOC Sciences provides one-stop site-specific conjugation services for amino acids, glycans, non-natural amino acids, and short peptide tags. In addition, cysteine conjugation, lysine conjugation, enzymatic conjugation, thio-engineered antibody can also be obtained quickly.
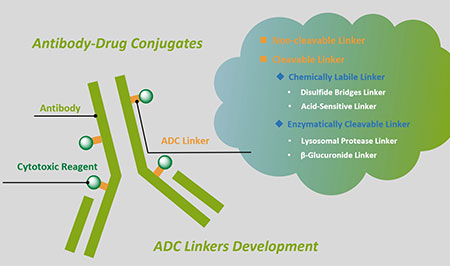
BOC Sciences offers a full range of linkers, including peptide linkers, PEG linkers, click chemistry, PROTAC linkers, non-cleavable linkers, etc. We also provide custom development services for chemically labile linkers and enzymatically cleavable linkers.