Linker-payload design is a critical aspect of antibody-drug conjugate (ADC) development, directly determining the stability, targeting ability, and therapeutic efficacy of the drug in vivo. With the advancement of targeted therapies and precise drug delivery technologies, scientists are increasingly focusing on the chemical compatibility, spatial configuration, and pharmacokinetic properties between the linker and the payload. A well-designed linker-payload not only enhances the cytotoxic efficiency of the drug but also reduces systemic side effects, providing a solid foundation for clinical development.
In the structure of an ADC, the linker-payload module is responsible for stably and controllably attaching the small-molecule drug (payload) to the antibody. The linker should remain stable in circulation to prevent premature drug release while being cleavable under specific intracellular conditions (such as pH, enzymes, or reducing environments) to release the active drug. The payload is typically a highly potent cytotoxic molecule, such as microtubule inhibitors (MMAE, DM1) or DNA-damaging agents (PBD, Calicheamicin).
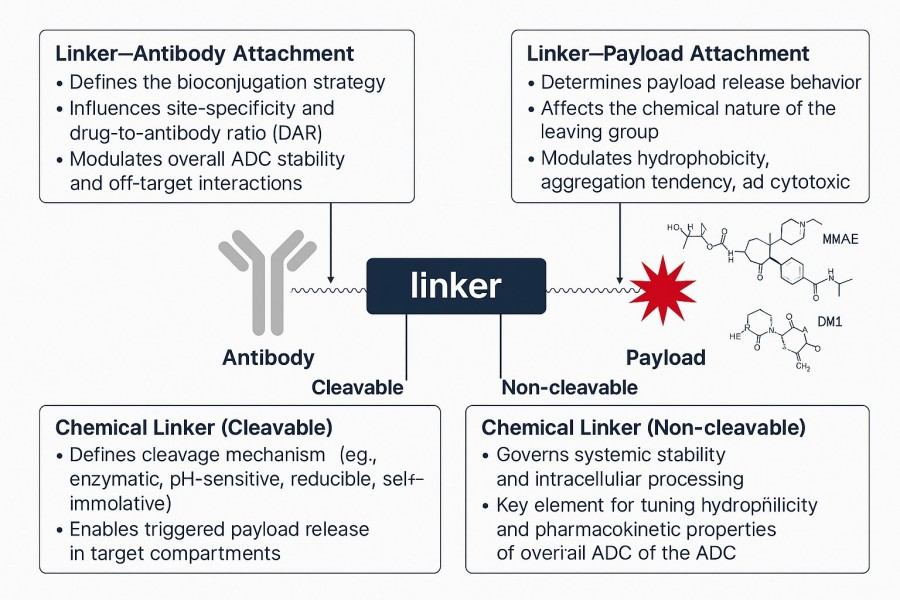 Fig. 1. The role and conjugation methods of linkers in ADC structures (BOC Sciences Authorized).
Fig. 1. The role and conjugation methods of linkers in ADC structures (BOC Sciences Authorized).
The rational design of the linker-payload system directly impacts:
Therefore, choosing the right linker-payload is not only a chemical synthesis issue but also a core aspect of pharmacology and biological system co-design.
A successful ADC must remain stable in systemic circulation, achieve precise release within target cells, and demonstrate potent cytotoxicity alongside favorable pharmacokinetics. When selecting a linker-payload, multiple factors including chemical properties, pharmacology, and biocompatibility need to be considered to achieve the optimal balance between stability, release efficiency, and biodistribution.
The primary role of the linker is to protect the payload from premature release in the bloodstream while allowing rapid cleavage in the target cell environment, ensuring drug selectivity and safety. An ideal linker possesses both "stable in circulation" and "cleavable in target cells" characteristics. Common design strategies include:
The payload is the core pharmacologically active component of an ADC. Its selection must balance cytotoxic potency, target specificity, and chemical modifiability. Typically, payloads need to be effective at sub-nanomolar levels to achieve cell-killing effects with minimal drug amounts. Depending on the mechanism of action, payloads can be classified as:
The DAR represents the average number of drug molecules attached per antibody and is a key parameter influencing ADC efficacy and safety. High-DAR ADCs exhibit stronger cytotoxicity but may cause protein aggregation, accelerated plasma clearance, and increased off-target toxicity. Low-DAR ADCs may show insufficient efficacy. Therefore, optimizing DAR aims to find the balance between activity and stability.
Traditional random conjugation methods often result in heterogeneous products and inconsistent DAR, affecting drug uniformity and safety. In contrast, site-specific conjugation enables precise modification at defined antibody sites, significantly improving product homogeneity and pharmacokinetic consistency. Common approaches include:
The physicochemical properties of the linker and payload significantly affect ADC behavior in vivo.
Optimizing the linker-payload combination can modulate ADC pharmacokinetics, resulting in longer half-life, higher tumor accumulation, and reduced systemic toxicity.
Discover our comprehensive collection of premium linker-payload solutions, featuring cleavable, non-cleavable, and modular designs. Engineered for optimal stability, controlled payload release, and seamless integration into cutting-edge research.
The compatibility between the linker and payload directly determines drug stability, release efficiency, and bioactivity. Ideal designs achieve not only efficient chemical conjugation but also compatibility in physiological environments and intact pharmacodynamic delivery. As ADC technology matures, payload-linker design has evolved from simple chemical connections to systematic strategies based on structural optimization, kinetic control, and molecular interaction regulation.
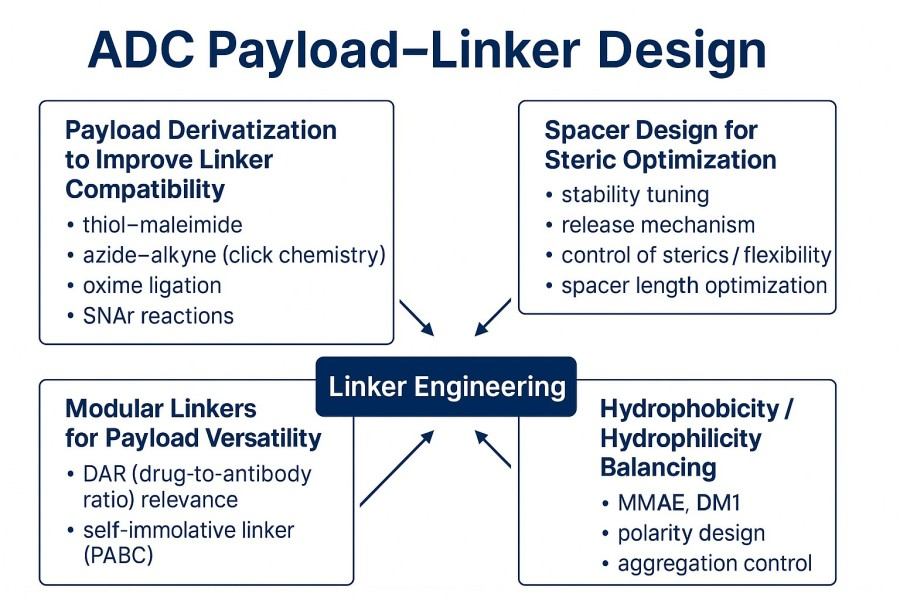 Fig. 2. ADC payload-linker design strategies (BOC Sciences Authorized).
Fig. 2. ADC payload-linker design strategies (BOC Sciences Authorized).
The chemical structures of payload molecules vary greatly, and their functional groups, reactivity, and solubility strongly influence conjugation efficiency. Mismatched reactivity between the payload and linker can lead to incomplete reactions, heterogeneous products, or payload inactivation. To address this, researchers often chemically modify payloads via derivatization to improve their compatibility with linkers.
For example, reactive groups (such as amino, carboxyl, thiol, or alkyne) can be introduced to enable specific conjugation to the linker, or the electronic structure and spatial configuration can be adjusted to optimize reaction rate and steric hindrance. Protective groups may also be used to shield critical active sites during conjugation, preserving payload bioactivity. This strategy improves chemical operability while enhancing solubility and stability in different solvent systems.
In a linker-payload system, the spacer ensures proper spatial configuration among the antibody, linker, and payload. Its length, flexibility, and polarity influence the overall ADC structure and bioactivity. Short spacers may bring the payload too close to the antibody surface, causing steric hindrance, restricted antigen recognition, or reduced release efficiency. Overly long spacers can increase molecular freedom, potentially causing nonspecific interactions.
Studies show that flexible spacers (e.g., PEG chains) can alleviate steric issues and provide optimal orientation for payload release. Rigid spacers (e.g., aromatic rings or alkyne linkages) are advantageous when precise positioning or prevention of folding is required. Spacer design should also consider chemical stability and biodegradability to maintain ADC performance in circulation and intracellular environments.
The concept of modular linkers is increasingly emphasized in ADC development. Traditional linkers are often customized for specific payloads or antibodies, lacking flexibility and scalability. Modular design introduces interchangeable functional modules within the linker, allowing rapid adaptation to different payload types or release mechanisms, thus improving drug screening and optimization efficiency.
For example, a modular linker may include a fixed unit for antibody conjugation, an adjustable core for stability, and a replaceable drug attachment end. By altering chemical bond types or reactive sites between modules, researchers can quickly evaluate the impact of different linker combinations on ADC properties. Modular structures also support multiple cleavable mechanisms (e.g., enzyme- and pH-sensitive dual responses) within the same linker system, providing precise pharmacological control for complex disease models.
The overall hydrophilic-hydrophobic balance of an ADC is a key factor in its in vivo behavior. Payloads are often highly hydrophobic, enhancing membrane permeability and cytotoxicity but potentially causing protein aggregation, nonspecific binding, and accelerated plasma clearance. To address this, linker design must achieve an appropriate balance between hydrophilicity and hydrophobicity.
In practice, researchers introduce hydrophilic segments (such as PEG, sulfonates, or amine groups) to reduce overall hydrophobicity, improving solubility and plasma stability. Conversely, for overly hydrophilic systems, adding hydrophobic units can enhance membrane penetration and payload release efficiency. This balance not only affects drug stability but also significantly influences immune recognition, cellular uptake pathways, and tissue distribution.
Through systematic regulation of linker polarity and molecular flexibility, ADC pharmacokinetics can be substantially optimized, achieving more favorable plasma half-life and tumor accumulation.
Even in the most advanced ADC development programs, matching the linker and payload still faces a series of technical and chemical challenges.
The DAR is a key parameter that measures the average number of drug molecules attached to each antibody, directly impacting the pharmacokinetics, toxicity, and efficacy of an ADC.
High DAR often increases the hydrophobicity of ADC molecules, leading to aggregation in vivo, rapid clearance, and potential hepatotoxicity. Conversely, low DAR may result in insufficient drug effect and reduced therapeutic efficacy.
During the linker-payload design phase, scientists typically consider the modification sites on the antibody surface, the physicochemical properties of the payload, and the linker's length and polarity to achieve an optimal DAR (commonly between 2 and 4) through precise chemical control. Additionally, site-specific conjugation techniques, such as enzyme-mediated conjugation or genetically encoded non-natural amino acids, can significantly improve DAR uniformity and reduce batch-to-batch variability.
Many potent cytotoxic drugs, such as auristatins and maytansinoids, are highly hydrophobic, posing challenges in ADC development. Hydrophobic payloads can enhance intermolecular interactions, leading to aggregation and affecting solubility and stability.
To address this, researchers commonly employ strategies such as:
These design strategies can effectively enhance ADC stability in circulation and prevent premature drug release or aggregation.
Different cytotoxic drug molecules exhibit variable stability and compatibility during chemical reactions. Some payloads are sensitive to reaction conditions, such as pH, temperature, or organic solvents, which can lead to partial degradation or loss of activity. For example, certain microtubule inhibitors or DNA-damaging agents may undergo structural breakdown under strong acidic or basic conditions, reducing their efficacy.
To mitigate these issues, researchers typically:
Optimizing reaction pathways can significantly improve the overall yield and bioactivity of the ADC.
Steric effects are a critical consideration when designing linker-payload systems. Short linkers or conjugation sites located too close to antibody active regions may impair antigen recognition or weaken Fc receptor binding, reducing ADC targeting and immune function. Additionally, bulky payloads may restrict conjugation efficiency or molecular conformation if spatial layout is not properly considered.
To prevent these issues, development teams often:
These approaches maintain antibody bioactivity while ensuring effective payload release and drug stability.
Linker-payload technology is not only central to ADC design but also a driving force for innovation in targeted therapies within biomedical research. With advances in conjugation chemistry and molecular engineering, this technology has expanded from traditional antibody-drug applications to oligonucleotides, antibody fragments, peptides, and multifunctional nanocarriers.
ADCs are the most mature and widely applied representation of linker-payload technology. Their core principle is to deliver potent cytotoxic drugs specifically to tumor cells using high-affinity antibodies, reducing systemic toxicity while enhancing therapeutic efficacy. Typical examples include:
AOCs represent the extension of linker technology to gene-level targeted therapies. Combining the tissue specificity of antibodies with the gene-regulatory ability of oligonucleotides (e.g., siRNA, ASO, mRNA), AOCs enable targeted modulation of disease-related gene expression. In AOCs, linkers must ensure stable chemical attachment and efficient intracellular cleavage to allow oligonucleotides to enter the cytoplasm and exert their effects. AOCs show great potential in genetic disorders, neurodegenerative diseases, and tumor gene silencing, with multiple candidates currently in clinical development.
FDCs use small antibody fragments, such as single-chain variable fragments (scFv), Fab, or VHH (nanobodies), conjugated to payloads. Compared with full-length ADCs, FDCs have lower molecular weight, faster tissue penetration, and shorter circulation half-life, making them ideal for tumor microenvironments, brain tissue, or concealed targets. However, the lack of an Fc region limits in vivo stability and circulation time, posing higher demands on linker-payload design. FDCs show promising applications in precision oncology, targeted radiopharmaceuticals, and imaging.
PDCs use short peptides as targeting ligands to deliver cytotoxic or therapeutic molecules to specific cells or tissues. Compared with ADCs, PDCs have simpler structures, easier synthesis, and good tissue penetration. Linkers in PDCs are crucial for controlling drug release rate, metabolic stability, and targeting selectivity. PDCs have been widely used in targeted cancer therapies, antimicrobial peptide delivery, and peptide vaccine development. Some PDC candidates, such as Melflufen, have demonstrated excellent antitumor activity in clinical studies.
BOC Sciences leverages comprehensive expertise in linker design, payload synthesis, integrated conjugation, and structural characterization to provide efficient, customizable linker-payload solutions. From early proof-of-concept to candidate screening and preclinical development, we offer end-to-end support from molecular design to functional validation, accelerating the development of ADCs and related precision therapeutics.
References
From cytotoxins to linkers, explore our cutting-edge products for your ADC project.
| Catalog | Name | CAS | Price |
| BADC-00014 | Mc-MMAF | 863971-19-1 | Bulk Inquiry |
| BADC-00029 | MC-Val-Cit-PAB-MMAE | 646502-53-6 | Bulk Inquiry |
| BADC-00009 | DM1-SMCC | 1228105-51-8 | Bulk Inquiry |
| BADC-00008 | Val-Cit-PAB-MMAE | 644981-35-1 | Bulk Inquiry |
| BADC-00606 | Deruxtecan | 1599440-13-7 | Bulk Inquiry |
| BADC-00020 | DM1-SMe | 138148-68-2 | Bulk Inquiry |
| BADC-00016 | Vat-Cit-PAB-MMAD | 1415329-13-3 | Bulk Inquiry |
| BADC-00010 | DM1-SPP | 452072-20-7 | Bulk Inquiry |
| BADC-00022 | DM3-SMe | 796073-70-6 | Bulk Inquiry |
| BADC-00021 | DM4-SMe | 796073-68-2 | Bulk Inquiry |
Explore our advanced tools and expertise for next-generation ADC research and development.
Potent cytotoxins designed for targeted antibody-drug conjugate therapy.
Stable, selective linkers enabling precise drug release in ADCs.
Integrated payload-linker solutions for efficient and targeted ADC delivery.
Targeted therapeutics combining antibodies with cytotoxic drugs for precision treatment.










