ADC linkers play a critical bridging role in antibody-drug conjugates (ADCs), directly connecting the antibody to the cytotoxic payload and significantly influencing drug delivery efficiency and targeting selectivity. An ideal ADC linker must maintain high stability in circulation to prevent premature payload release while enabling precise and controllable drug release within target cells. Different types of linkers, such as non-cleavable linkers, enzyme-cleavable linkers, pH-sensitive linkers, or GSH-sensitive linkers, have chemical properties, lengths, hydrophobicity, and conjugation modes that markedly impact the efficacy and safety of ADCs. This article systematically analyzes how ADC linkers regulate drug delivery and selectivity from the perspectives of linker design principles, target cell specificity, circulation stability, and triggered release mechanisms.
In an ADC system, the linker is the core chemical module connecting the antibody and the payload, directly influencing ADC stability, efficacy, and safety. Rational ADC linker design not only ensures payload integrity in circulation but also enables precise release within tumor cells, enhancing therapeutic selectivity while minimizing toxicity. With the continuous advancement of current ADC linker chemistry, researchers can optimize linker functional groups, hydrophobicity, and cleavable properties to improve payload delivery efficiency and broaden the therapeutic window.
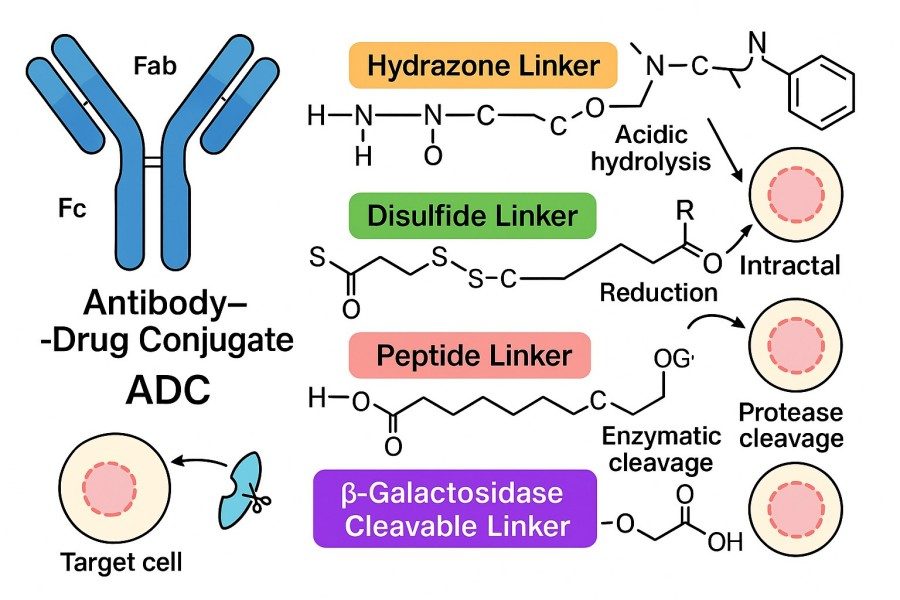 Fig. 1. Current ADC linker chemistry (BOC Sciences Authorized).
Fig. 1. Current ADC linker chemistry (BOC Sciences Authorized).
ADCs are precision medicines that couple monoclonal antibodies with highly potent cytotoxic drugs (payloads) via chemical linkers. The therapeutic effect of an ADC depends not only on the antibody's targeting and the payload's potency but also heavily on the design and performance of the ADC linker. The linker acts as a bridge, ensuring safe transport of the payload to tumor cells and its release at the right time and location. The type, stability, and functionality of an ADC linker directly influence the therapeutic window, in vivo circulation half-life, and off-target toxicity. Advances in ADC linker chemistry focus on optimizing payload release efficiency while minimizing premature release in plasma.
The ADC linker is the central chemical module connecting the antibody and payload, and its chemical properties and spatial structure determine the overall physicochemical characteristics of the ADC. The linker–payload–antibody interface affects not only the conformational stability of the ADC but also the binding kinetics between the antibody and the target antigen. Rational linker design can reduce payload solubility issues, improve ADC stability in circulation, and ensure precise drug release within the tumor microenvironment.
The choice of ADC linker directly affects the width of the therapeutic window. An overly stable linker may result in insufficient payload release, reducing ADC efficacy, while an overly labile linker may release the payload prematurely in plasma, increasing systemic toxicity. By precisely controlling the chemical structure, hydrophobicity or hydrophilicity, and reaction sensitivity of the linker, researchers can achieve an optimal balance between circulation stability and tumor-specific drug release, thereby broadening the therapeutic window of ADCs.
| Linker Type | Description |
| Acid Cleavable Linkers | Provide expert design and development support for acid-sensitive linkers to achieve precise payload release in target environments. |
| Disulfide Linkers | Offer customized strategies to optimize disulfide linker stability and controlled intracellular drug release. |
| Cathepsin B Cleavable Linkers | Deliver specialized solutions for enzymatically cleavable linkers activated by Cathepsin B in tumor cells. |
| Phosphatase Cleavable Linkers | Enable tailored design and synthesis of linkers responsive to phosphatase activity for selective payload delivery. |
| Sulfatase Cleavable Linkers | Support development of sulfatase-sensitive linkers for precise, enzyme-triggered drug release. |
| β-Galactosidase Cleavable Linkers | Provide capability in designing linkers that release payloads upon β-galactosidase activation. |
| β-Glucuronidases Cleavable Linkers | Facilitate the creation of linkers cleavable by β-glucuronidase to ensure controlled release in target tissues. |
The greatest advantage of ADCs is their ability to selectively kill tumor cells, and the linker plays a central role in payload activation, endocytosis, and lysosomal release. Different types of ADC cleavable linkers can trigger payload release in a target cell-specific manner, reducing off-target toxicity in blood and non-target cells. Through site-specific conjugation and synchronization of linker–antigen binding, researchers can further optimize ADC targeting.
The core advantage of ADCs lies in precise tumor cell killing, and the linker is critical in controlling the timing and location of payload activation. Enzyme-sensitive linkers cleave specifically under tumor-associated enzymes, enabling targeted payload release and avoiding non-specific activation in plasma. pH-sensitive linkers utilize the acidic tumor microenvironment or lysosomal pH to trigger payload release for precise control. GSH-sensitive linkers rely on intracellular reducing environments to cleave disulfide bonds, achieving efficient payload release within target cells and significantly improving ADC selectivity and safety.
After ADC internalization into target cells, the linker must efficiently release the payload within endosomes and lysosomes; otherwise, drug efficacy decreases dramatically. Linker length, hydrophobicity, rigidity, and spatial conformation affect ADC endocytosis efficiency and endosomal trafficking. Peptide linkers provide enzyme-cleavable functionality while modulating payload release rates, whereas PEG linkers improve solubility and reduce non-specific aggregation. Proper linker design optimizes endosomal release efficiency and increases the proportion of active payload delivered within target cells.
Inappropriate linkers may cause premature payload release in circulation or non-target cells, increasing systemic toxicity and side effects. Utilizing enzyme-cleavable, pH-sensitive, or GSH-sensitive linkers combined with site-specific conjugation allows precise control of payload release location. Selecting appropriate linker chemistry and conjugation sites ensures target-specific payload release within tumor cells, significantly reducing off-target toxicity—a crucial strategy for maximizing ADC efficacy while maintaining safety.
ADC linker design must consider not only payload release but also synchronization with antibody–antigen binding kinetics. Overly long or flexible linkers may enhance antibody reachability but reduce binding stability and payload release efficiency. Optimizing linker length, rigidity, and conformation ensures effective payload release while maintaining high antibody–antigen affinity. This approach balances targeting and efficacy, supporting precision therapy with ADCs.
To achieve precise targeted release, ADCs often rely on stimuli-sensitive linkers that trigger payload release in specific environments. ADC cleavable linkers respond to enzymes, pH, or reducing conditions, ensuring payload release only within tumor cells or designated microenvironments. By optimizing the chemical properties of enzyme-cleavable, pH-sensitive, and GSH-sensitive linkers, researchers can enhance payload release efficiency and ADC selectivity.
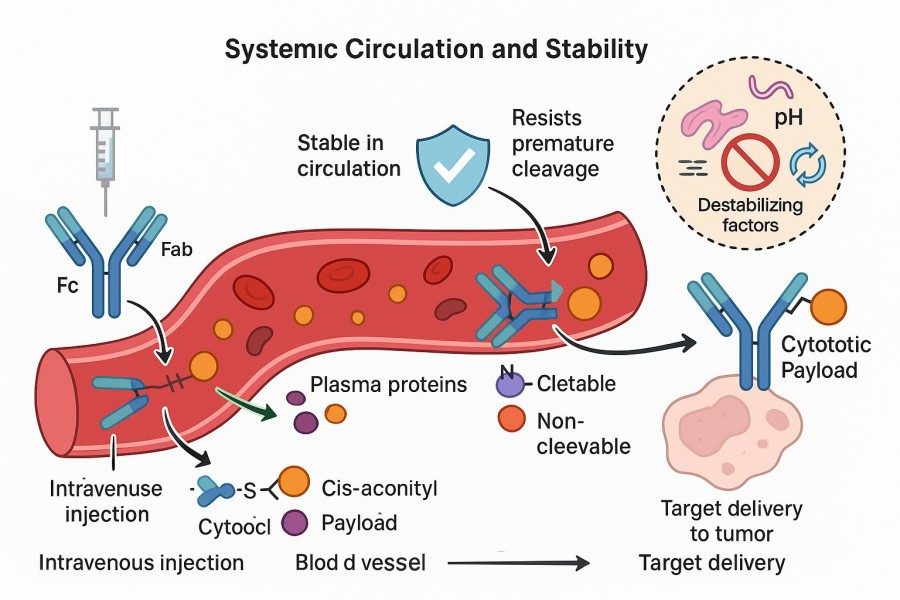 Fig. 2. Systemic circulation and stability of linkers (BOC Sciences Authorized).
Fig. 2. Systemic circulation and stability of linkers (BOC Sciences Authorized).
ADC linkers must remain highly stable in circulation to prevent premature payload release before reaching target cells, which could increase systemic toxicity. Highly stable linkers, such as non-cleavable linkers or optimized enzyme-cleavable linkers, maintain ADC integrity in plasma and extend the therapeutic window. Rational ADC linker stability protects payload activity and ensures effective delivery of the antibody-drug conjugate in circulation. Precise control of linker chemical structure and functional groups further enhances plasma stability and drug safety.
Premature payload release in circulation is one of the main challenges in ADC development, significantly reducing efficacy and increasing side effects. Using hydrazone, disulfide, or PEG linkers, and optimizing linker hydrophobicity and functional groups, can effectively minimize non-specific payload release in plasma. Coupled with site-specific conjugation and linker modification strategies, ADC stability in circulation is further enhanced, ensuring payload release only in target cells or specific microenvironments and improving ADC therapeutic selectivity and safety.
Chemical modifications of ADC linkers significantly affect pharmacokinetic (PK) and pharmacodynamic (PD) profiles. Introducing hydrophilic or hydrophobic groups into peptide linkers can improve ADC solubility and stability in plasma while optimizing tissue distribution and targeted delivery. For PEG linkers, adjusting polyethylene glycol molecular weight can extend circulation half-life and improve tumor penetration, enhancing payload release efficiency and ADC efficacy. Rational linker modification strategies achieve optimal PK/PD balance in vivo, boosting overall drug performance.
Linker hydrophobicity and hydrophilicity are key factors influencing ADC performance. Hydrophobic linkers enhance payload-antibody binding efficiency, but excessive hydrophobicity may cause aggregation, precipitation, or non-specific adsorption, increasing blood toxicity. Hydrophilic linkers improve ADC solubility, enhance plasma circulation stability, and help reduce immunogenicity and non-target cell uptake. Proper adjustment of linker hydrophobicity can enhance payload delivery efficiency while optimizing overall ADC safety and efficacy.
To achieve precise targeted release, ADCs often rely on stimuli-sensitive linkers that trigger payload release in specific environments. ADC cleavable linkers can respond to enzymes, pH, or reducing conditions, ensuring that the payload is released only within tumor cells or designated microenvironments. By optimizing the chemical properties of enzyme-cleavable, pH-sensitive, and GSH-sensitive linkers, researchers can enhance payload release efficiency and improve ADC selectivity.
Enzyme-sensitive linkers enable selective payload release in ADCs through the action of specific enzymes, with cathepsin B–sensitive peptide linkers being the most common type. These linkers are cleaved by specific enzymes within the lysosomes of tumor cells, such as Val-Cit and Phe-Lys linkers, widely used in FDA-approved ADCs including Brentuximab Vedotin and Polatuzumab Vedotin. Beyond cathepsin B, other overexpressed enzymes in the tumor microenvironment, such as β-glucuronidase and phosphatases, can also serve as triggers to efficiently release payloads in targeted microenvironments. By optimizing peptide sequences, linker length, solubility, and spatial conformation, precise payload release within target cells can be achieved.
pH-sensitive linkers utilize acidic environments to trigger payload release, enabling targeted delivery of ADCs. Acid-labile linkers, such as hydrazone and cis-aconityl linkers, cleave in lysosomes or acidic tumor microenvironments while remaining stable at blood pH 7.4, significantly reducing non-specific payload release in circulation. Optimizing linker chemical structures ensures rapid payload release in acidic conditions, enhancing tumor cell killing while maintaining stability in neutral blood environments.
GSH-sensitive linkers rely on disulfide bond cleavage to achieve selective intracellular payload release. The high glutathione concentration and reducing environment in tumor cells provide a differential trigger mechanism, allowing disulfide linkers to cleave efficiently inside target cells while remaining stable in plasma. Compared to enzyme-cleavable and pH-sensitive linkers, GSH-sensitive linkers do not require specific enzymes or acidic conditions, offering broader applicability and stability. Rational design of disulfide bond structures and the linker chemical environment can further optimize payload release rates and ADC selectivity.
Efficient payload delivery requires balancing linker stability in circulation with intracellular release efficiency while considering manufacturability. Site-specific conjugation, linker–payload ratio optimization, and functional group design are key factors to enhance ADC efficacy. Optimized ADC linker design can achieve higher drug–antibody ratios (DAR), reduce aggregation risks, and improve PK/PD profiles.
An ideal ADC linker must maintain high stability in circulation while efficiently releasing the payload in target cells for precise therapy. Chemical modifications, peptide sequence optimization, and PEGylation can tune the balance between linker stability and reactivity, preventing premature release or insufficient payload activation. Linker spatial conformation and functional group design are critical to maintaining payload activity in circulation while enabling effective intracellular release. Overall, a well-balanced stability and reactivity profile directly impacts ADC efficacy and safety.
Site-specific conjugation techniques, such as lysine-linked ADCs and cysteine-engineered ADCs, significantly improve payload conjugation uniformity and reduce batch-to-batch variability. Precisely controlling linker–payload attachment sites optimizes ADC pharmacokinetics and tissue distribution. Different conjugation sites can influence payload release efficiency and antibody binding properties, making careful selection of sites and linker chemistry essential. This approach enhances both ADC stability and therapeutic potency and targeting.
The DAR is a crucial parameter affecting ADC efficacy and toxicity. High DAR increases payload cytotoxicity but may lead to plasma aggregation and off-target toxicity. Low DAR reduces side effect risks but may compromise therapeutic efficacy. By optimizing linker structure and conjugation strategy, an optimal DAR can be achieved to maximize efficacy while maintaining safety, ensuring ADCs perform optimally in vivo.
Industrial production of ADC linkers requires chemical feasibility, reproducibility, and GMP compliance to meet clinical and commercial demands. Different linker types, such as disulfide, hydrazone, peptide, and PEG linkers, must consider manufacturability and batch stability during design. Standardized production processes and rigorous quality control are essential to maintain linker structural integrity and functionality. Proper design and manufacturing workflows ensure high-performance, stable ADCs in large-scale production, supporting clinical application requirements.
Specific ADC cases illustrate the roles of different linker types in drug delivery and selectivity. T-DM1's non-cleavable linker, Brentuximab Vedotin's enzyme-cleavable peptide linker, and emerging dual-responsive linker ADCs demonstrate the critical role of linkers in optimizing efficacy while reducing toxicity.
Trastuzumab Emtansine (T-DM1) uses an SMCC non-cleavable linker to stably conjugate the MCC-DM1 payload to the trastuzumab antibody. The non-cleavable linker maintains high stability in circulation, reducing off-target toxicity and extending the therapeutic window. Payload release occurs gradually through antibody degradation in lysosomes, achieving precise targeting and controlled drug release. This design ensures plasma stability while providing safe and effective targeted therapy.
Brentuximab Vedotin employs a Val-Cit–PABC enzyme-cleavable peptide linker to conjugate the MMAE payload to the antibody. The linker is specifically cleaved by cathepsin B in tumor cell lysosomes, enabling efficient payload release. This design allows rapid tumor cell killing while maintaining stability in circulation, highlighting the advantages of enzyme-cleavable linkers in enhancing ADC selectivity and efficacy.
From cytotoxins to linkers, explore our cutting-edge products for your ADC project.
| Catalog | Name | CAS | Price |
| BADC-00008 | Val-Cit-PAB-MMAE | 644981-35-1 | Bulk Inquiry |
| BADC-00369 | Fmoc-Val-Cit-PAB-PNP | 863971-53-3 | Bulk Inquiry |
| BADC-00013 | MC-Val-Cit-PAB-MMAF | 863971-17-9 | Bulk Inquiry |
| BADC-00629 | MC-Val-Cit-PAB-Auristatin E | 2055896-77-8 | Bulk Inquiry |
| BADC-00370 | Phe-Lys(Fmoc)-PAB | 2149584-03-0 | Bulk Inquiry |
| BADC-00371 | Phe-Lys(Trt)-PAB | 1116085-99-4 | Bulk Inquiry |
| BADC-00968 | MC-Val-Cit-PAB | 159857-80-4 | Bulk Inquiry |
| BADC-00443 | Mal-PEG5-NHS | 1315355-92-0 | Bulk Inquiry |
| BADC-00447 | Mal-amido-PEG2-NHS | 955094-26-5 | Bulk Inquiry |
| BADC-00448 | Mal-PEG-NHS | 1260092-50-9 | Bulk Inquiry |
| BADC-00029 | MC-Val-Cit-PAB-MMAE | 646502-53-6 | Bulk Inquiry |
Explore our advanced tools and expertise for next-generation ADC research and development.
Potent cytotoxins designed for targeted antibody-drug conjugate therapy.
Stable, selective linkers enabling precise drug release in ADCs.
Integrated payload-linker solutions for efficient and targeted ADC delivery.
Targeted therapeutics combining antibodies with cytotoxic drugs for precision treatment.










