Acid labile linkers are a critical component in antibody-drug conjugates (ADCs) for achieving precise drug release. These pH-sensitive cleavable linkers remain stable in plasma while rapidly cleaving in the acidic environment of tumor cells to release cytotoxic payloads. This mechanism improves the therapeutic index of ADCs and reduces off-target toxicity. This article systematically explores the definition, common chemical structures, mechanisms of action, and development challenges of acid labile linkers. It also discusses optimization strategies and clinical examples, providing ADC researchers with design and application references to support efficient and precise drug delivery.
In the design of ADCs, linkers are one of the core elements for achieving precise drug release. Acid labile linkers (acid cleavable linkers) respond specifically to acidic environments, remaining stable in plasma and efficiently releasing drugs within tumor cells, making them a central focus in pH-sensitive cleavable linker design.
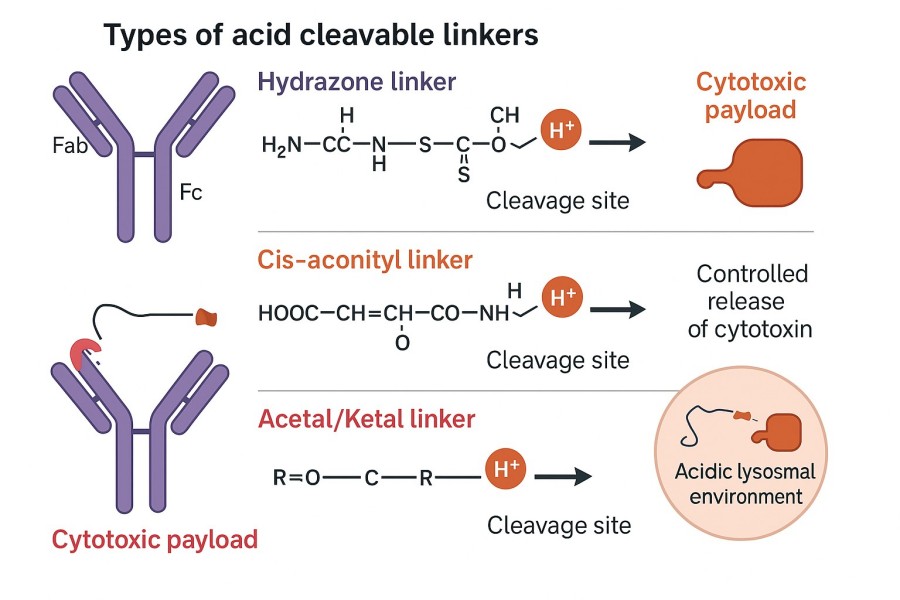 Fig. 1. Types of acid cleavable linker (BOC Sciences Authorized).
Fig. 1. Types of acid cleavable linker (BOC Sciences Authorized).
Acid labile linkers are chemical structures that cleave under acidic conditions, making them particularly suitable for the precise release of payloads in ADCs. Plasma pH is approximately 7.4, while the pH within lysosomes and endosomes of tumor cells typically ranges from 4.5 to 6.5. This pH difference provides selective cleavage conditions for acid cleavable linkers. Key characteristics include:
Common acid labile linkers feature diverse chemical structures and functional groups. Each type performs differently in pH-sensitive cleavable linker design, and selection must consider payload properties, plasma stability, and release kinetics.
Hydrazone linkers are among the most widely used acid labile linkers. They are typically formed by condensation of aldehydes or ketones with hydrazine groups (–NH–NH–). Acid labile hydrazone linkers efficiently release payloads through hydrolysis in acidic environments (pH 4.5–6.5). By introducing electron-donating or electron-withdrawing groups, their pH sensitivity and plasma stability can be finely tuned—stable in circulation while rapidly degrading inside target cells. Mature and scalable synthetic routes make hydrazone linkers suitable for large-scale GMP production. They are widely applied in ADCs such as the FDA-approved Mylotarg®. Current research focuses on developing more acid-sensitive hydrazone linkers to further improve plasma stability, minimize premature drug release, and enhance the therapeutic index.
Cis-aconityl linkers are formed by conjugating cis-aconitic acid with amines to create acid-sensitive amide bonds, releasing payloads via acid-catalyzed hydrolysis. Compared to hydrazone linkers, cis-aconityl structures offer improved plasma stability, making them ideal for ADCs requiring longer circulation times. Their pH-responsive behavior is predictable, and release rates are relatively stable, reducing non-specific release. However, synthesis is more complex, and compatibility with certain payloads may require optimization, increasing early development workload. Nevertheless, cis-aconityl remains a strong candidate for acid cleavable linker antibody design when extended in vivo circulation is desired.
Acetal and ketal structures are classic pH-sensitive cleavable linkers that rapidly hydrolyze under acidic conditions to produce corresponding alcohols and carbonyl compounds. They are particularly suitable for ADCs that require fast payload release within tumor cells, allowing cytotoxic effects to occur shortly after entry into endosomes or lysosomes. Hydrolysis rates can be precisely adjusted through steric modifications or substituent variation, providing highly controllable release kinetics. This makes them valuable for constructing efficient acid labile linkers for antibody-drug conjugates.
Orthoesters are versatile acid-labile linkers for antibody-drug conjugates. They decompose in acidic environments to form esters and alcohols, and byproducts can be designed as non-toxic or functional molecules. Orthoesters offer exceptional structural tunability, allowing precise control of pH responsiveness to match intracellular conditions and payload release requirements. They are compatible with both hydrophilic and hydrophobic payloads, and can be combined with other linkers to achieve dual-triggered drug release. In multifunctional acid cleavable linker antibody formulations, orthoester structures are often used as core design elements, providing rich chemical flexibility for personalized ADC development.
| Linker Type | Description |
| Acid Cleavable Linkers | Enables customized acid-sensitive linker design and optimization for efficient, controlled drug release. |
| Disulfide Linkers | Supports versatile disulfide linker development, optimizing stability and reductive release kinetics. |
| Cathepsin B Cleavable Linkers | Designs and synthesizes linkers specifically cleavable by Cathepsin B to enhance ADC targeting. |
| Phosphatase Cleavable Linkers | Provides development and evaluation of phosphatase-cleavable linkers to ensure targeted release. |
| Sulfatase Cleavable Linkers | Supports sulfatase-cleavable linker design and validation for precise drug delivery. |
| β-Galactosidase Cleavable Linkers | Develops linkers cleavable by β-galactosidase to optimize drug release efficiency. |
| β-Glucuronidases Cleavable Linkers | Offers β-glucuronidase-sensitive linker design and functional evaluation for highly selective release. |
The core principle of acid labile linkers is cleavage of acid-sensitive chemical bonds within the low-pH environment of tumor cells, enabling precise payload release. Different chemical structures significantly influence release rates and stability, making a deep understanding of their mechanisms crucial for ADC optimization.
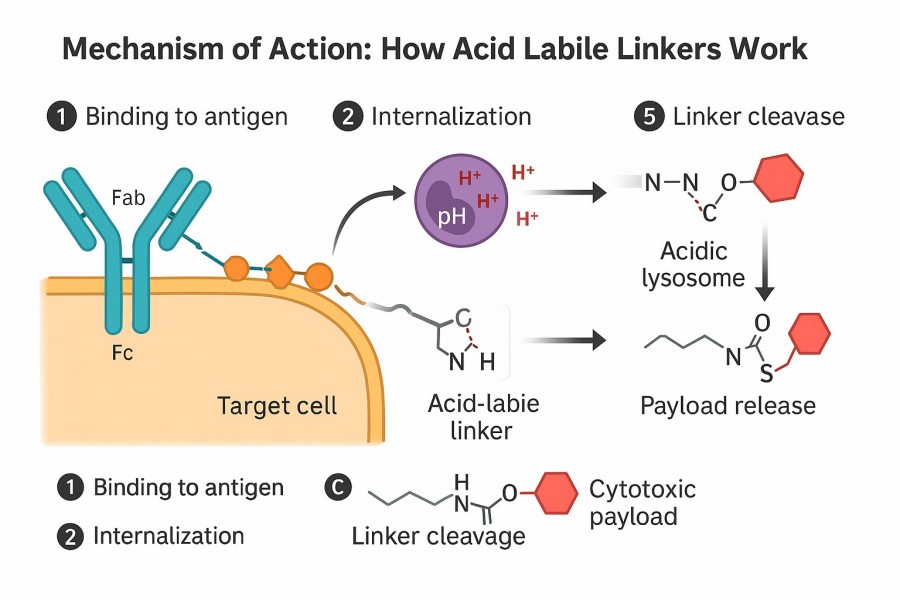 Fig. 2. pH-cleavable linker in lysosomes (BOC Sciences Authorized).
Fig. 2. pH-cleavable linker in lysosomes (BOC Sciences Authorized).
After ADC internalization via receptor-mediated endocytosis, the conjugates are transported to acidic organelles such as endosomes and lysosomes, where pH values typically range from 4.5 to 6.0. This acidity triggers chemical cleavage of acid labile linkers, releasing the payload precisely. For example, acid-sensitive hydrazone linkers undergo hydrolysis under these conditions, rapidly liberating cytotoxic drugs to kill tumor cells.
The release kinetics of acid labile linkers depend heavily on their chemical structure and functional modifications. Introducing electron-donating groups into hydrazone linkers can accelerate hydrolysis, enabling faster payload release, whereas electron-withdrawing groups can slow cleavage, enhancing plasma stability and reducing off-target release. This chemical tunability allows researchers to match the release profile to the therapeutic requirements of different ADC projects.
Unlike disulfide linkers, which rely on intracellular glutathione (GSH) levels, acid cleavable linkers are entirely triggered by acidic environments, making them less dependent on variable intracellular redox states. In tumors with fluctuating GSH concentrations, pH-sensitive linkers can offer more stable and predictable release. Compared with enzymatically cleavable linkers, acid labile linkers provide greater structural control through functional group modifications and steric adjustments, offering wider optimization potential for ADC development.
Although acid labile linkers provide ADCs with precise drug release and high selectivity, they still face multiple challenges during development. Researchers must balance plasma stability with intracellular release rates, control non-specific early payload release, and address aggregation issues caused by linker hydrophobicity.
One of the biggest challenges in developing acid labile linkers is achieving both plasma stability and efficient cleavage within tumor cells. An ideal acid cleavable linker antibody should remain intact during circulation to prevent premature payload release in normal tissues, reducing systemic toxicity. However, once inside the acidic environment of tumor cells, the linker must rapidly cleave to ensure effective drug delivery. Researchers often employ functional group modifications or molecular conformation optimization to achieve this delicate balance.
In the use of pH-sensitive hydrazone linkers, controlling non-specific hydrolysis is central to reducing adverse effects. Certain tissues or pathological conditions in the human body may have slightly acidic pH, and improper linker design could trigger hydrazone linker cleavage before reaching the tumor, causing premature payload release. This not only diminishes ADC therapeutic efficacy but may also induce serious side effects. Optimizing acid-sensitive hydrazone linker stability through electronic effects, steric hindrance, and protective structural strategies is therefore a critical aspect of development.
The hydrophobicity of acid labile linkers can significantly impact ADC pharmacokinetics and formulation stability. Excessive hydrophobicity in the linker-payload conjugate can lead to aggregation in blood, altering distribution and accelerating clearance. To address this, researchers often introduce hydrophilic groups into the linker structure or design acid cleavable linkers with polar side chains to improve solubility and dispersibility. This strategy not only reduces aggregation risk but also enhances ADC circulation half-life and therapeutic performance.
To fully leverage the potential of acid labile linkers, researchers need to optimize molecular structure, functional group modifications, and integration strategies with different payloads. Fine-tuning linker length, spatial configuration, and hydrolysis rates can significantly improve ADC efficacy and safety.
In ADC design, the length and flexibility of acid labile linkers are critical factors affecting conjugation efficiency and release performance. Proper adjustment of linker length and spatial structure can reduce steric hindrance, improve payload-antibody binding efficiency, and optimize intracellular release rates. Introducing flexible spacers further enhances spatial compatibility with diverse payloads, allowing acid cleavable linker antibodies to accommodate complex molecular structures and improve overall ADC efficacy and stability.
For pH-sensitive hydrazone linker design, introducing different substituents or modulating electronic effects enables precise control over hydrazone linker cleavage rates. Electron-donating groups accelerate acid hydrolysis for faster payload release, while electron-withdrawing groups slow cleavage, enhancing plasma stability. This strategy allows tailoring of release profiles according to therapeutic windows and payload characteristics, optimizing efficacy while ensuring safety.
The physicochemical properties of different payloads (hydrophilicity, hydrophobicity, molecular weight) impose specific design requirements on acid cleavable linkers. Customizing linker structures for each payload type ensures stability in plasma while enabling efficient release under acidic conditions. Properly matching linker and payload properties maximizes ADC potency, reduces aggregation and non-specific release risks, and enables efficient, safe precision drug delivery.
Acid labile linkers have achieved success in multiple clinical ADCs and show broad potential in preclinical research. Understanding these examples helps researchers evaluate the performance of acid labile hydrazone linkers and other acid-sensitive linkers in various applications.
Clinically, acid labile linkers have demonstrated remarkable results. The FDA-approved Mylotarg® (gemtuzumab ozogamicin) utilizes an acid-sensitive hydrazone linker to efficiently release the DNA-damaging agent calicheamicin within lysosomes, achieving precise targeting of leukemia cells. This linker design exemplifies the advantages of pH-sensitive hydrazone linkers in ADCs—stable in plasma while rapidly cleaving inside tumor cells—enhancing the therapeutic index and reducing systemic toxicity. Mylotarg®'s success provides valuable insights and a reference model for subsequent ADC linker development.
In preclinical research, several ADC projects are evaluating modified hydrazone linker aldehydes to further optimize plasma stability and hydrazone linker cleavage rates. These new acid-sensitive linkers maintain payload activity while responding more precisely to the low pH within tumor cells, reducing side effects from non-specific release. Through structural modifications and functional group adjustments, researchers can control the release kinetics of acid cleavable linkers to be compatible with various payload types, including DNA toxins, small-molecule chemotherapeutics, or protein toxins. These preclinical cases demonstrate the potential of acid labile linkers to enhance ADC efficacy and safety.
Successful application of acid labile linkers requires advanced chemical design, conjugation strategies, and production capabilities. BOC Sciences provides leading linker support services across ADC development, covering design, custom synthesis, conjugation optimization, and stability assessment. With extensive chemical synthesis experience and strict quality control, we offer tailored solutions for different payloads and target requirements. Combined with our in-stock linker library and customization capabilities, we help clients accelerate ADC development, improving candidate stability, uniformity, and efficacy, thereby achieving efficient and safe R&D outcomes.
From cytotoxins to linkers, explore our cutting-edge products for your ADC project.
| Catalog | Name | CAS | Price |
| BADC-00374 | m-C-tri(CH2-PEG1-NHS ester) | 173414-89-6 | Bulk Inquiry |
| BADC-00511 | DUPA(OtBu)-OH | 1026987-94-9 | Bulk Inquiry |
| BADC-01916 | 20-(tert-Butoxy)-20-oxoicosanoic acid | 683239-16-9 | Bulk Inquiry |
| BADC-00615 | JUN68744 | 1703768-74-4 | Bulk Inquiry |
| BADC-00982 | Fmoc-N-amido-PEG2-acetic acid | 166108-71-0 | Bulk Inquiry |
| BADC-01586 | Fmoc-Gly-Gly-Gly-Gly-OH | 1001202-16-9 | Bulk Inquiry |
| BADC-01140 | DSP Crosslinker | 57757-57-0 | Bulk Inquiry |
| BADC-00886 | N-Hydroxysulfosuccinimide sodium | 106627-54-7 | Bulk Inquiry |
| BADC-01339 | AEEA-AEEA | 1143516-05-5 | Bulk Inquiry |
| BADC-00622 | Boc-Gly-Gly-Phe-Gly-OH | 187794-49-6 | Bulk Inquiry |
Explore our advanced tools and expertise for next-generation ADC research and development.
Potent cytotoxins designed for targeted antibody-drug conjugate therapy.
Stable, selective linkers enabling precise drug release in ADCs.
Integrated payload-linker solutions for efficient and targeted ADC delivery.
Targeted therapeutics combining antibodies with cytotoxic drugs for precision treatment.










