ADC linkers are a critical component of antibody-drug conjugates (ADCs). They not only connect the antibody to the active drug but also directly influence the ADC's in vivo stability, drug release efficiency, and therapeutic index. Selecting an appropriate linker requires a comprehensive evaluation of the drug's chemical properties, the antibody structure, and the target tissue environment. Different types of linkers, such as cleavable and non-cleavable linkers, or functionalized specialized linkers, each offer advantages in drug release mechanisms, blood stability, and off-target toxicity control. This guide systematically analyzes the classification, design strategies, and optimization methods of ADC linkers, helping researchers understand the characteristics, application scenarios, and key considerations for each type, thereby enabling efficient and safe ADC development and enhancing the success rate of translational research and clinical applications.
In ADCs, linkers serve as the crucial bridge that effectively connects antibodies to cytotoxic drugs. The design of the linker not only affects the stability of the ADC but also directly determines the efficiency of drug release in vivo and the therapeutic outcome. A well-designed linker ensures targeted drug delivery while minimizing non-specific toxicity, thereby improving the ADC's therapeutic index. Therefore, for any ADC development team, a deep understanding of the linker's chemical properties, functional characteristics, and compatibility with both the antibody and the drug is central to achieving efficient ADC development.
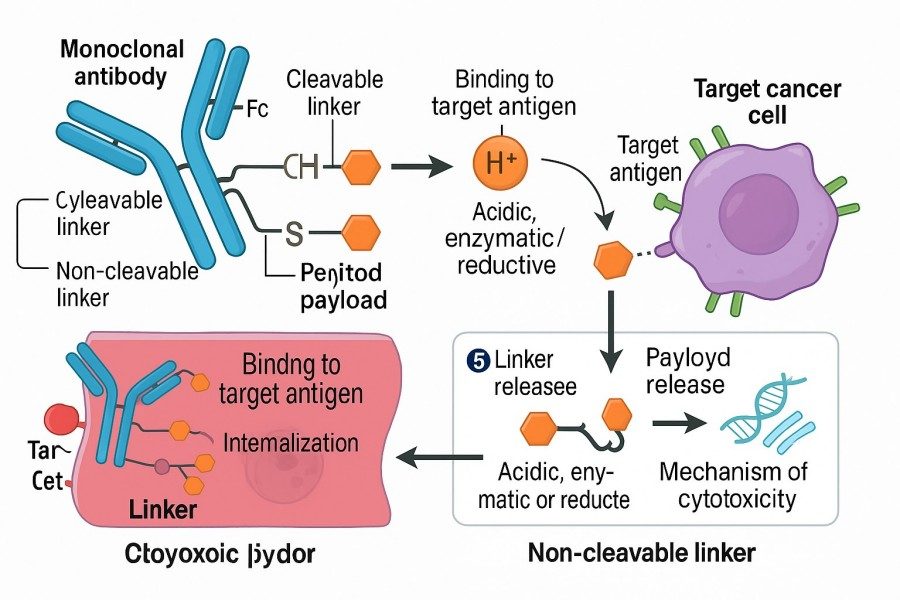 Fig. 1. Antibody-drug conjugate mechanism of action (BOC Sciences Authorized).
Fig. 1. Antibody-drug conjugate mechanism of action (BOC Sciences Authorized).
Linkers in ADCs are more than a simple chemical bridge; they determine the overall stability, drug release efficiency, and therapeutic selectivity of the ADC molecule. Specifically, linkers must fulfill the following key functions:
Designing and selecting ADC linkers presents multiple challenges, requiring research teams to balance chemical, pharmacological, and biological considerations:
ADC linkers can be divided into cleavable and non-cleavable categories, each offering advantages in mechanism, drug release, and clinical applications. Choosing the appropriate linker type requires careful consideration of the drug's properties, the antibody's characteristics, and the intended therapeutic mechanism. Understanding the features of each linker type helps achieve an optimal balance between efficacy and safety in ADC development.
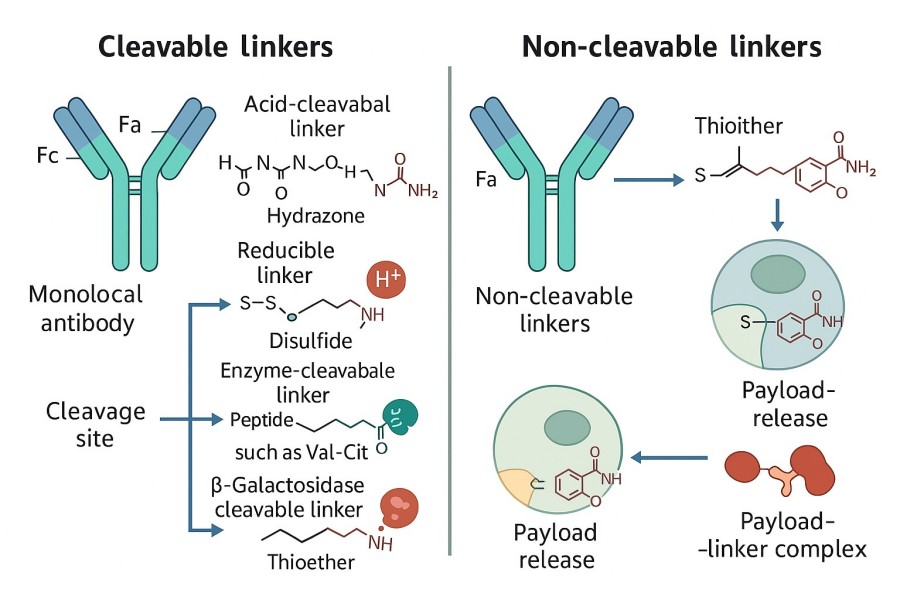 Fig. 2. ADC linker classification (BOC Sciences Authorized).
Fig. 2. ADC linker classification (BOC Sciences Authorized).
Cleavable linkers: Designed to release drugs under specific physiological conditions, such as acidic pH (lysosomes), highly reducing environments (intracellular glutathione), or specific enzymatic cleavage. Their advantage lies in enabling rapid, precise drug release within target cells, enhancing the ADC's antitumor effect. Typical cleavable linkers include peptide-based linkers and disulfide linkers, which allow controlled release via enzymatic or redox triggers. Cleavable linkers are suitable for ADCs requiring fast intracellular drug release but must be carefully designed to avoid premature release in circulation.
Non-cleavable linkers: Highly stable in blood and the body, drug release primarily occurs after antibody degradation, forming drug-antibody complexes or metabolites. Their advantages include high circulatory stability and low off-target toxicity, making them suitable for ADCs requiring sustained release or drugs sensitive to blood exposure. Common non-cleavable linkers include maleimide and SMCC linkers. Although their release is slower, they enhance overall ADC safety and are ideal for developing drugs with longer in vivo half-lives.
Selecting the appropriate linker type is a critical step in ADC development, requiring careful consideration of the drug's chemical properties, antibody structural features, and the intended in vivo release mechanism.
| Consideration | Key Features | Suitable Linker Types | Notes |
| Payload Characteristics | Polarity, hydrophilicity/hydrophobicity, chemical stability, drug release mechanism | PEGylated linkers, cleavable linkers (hydrazone, disulfide), non-cleavable linkers (SMCC, maleimide) | Hydrophobic drugs may aggregate; PEGylated or non-cleavable linkers improve solubility and stability. Acid- or reduction-sensitive drugs are suitable for cleavable linkers for targeted release. |
| Antibody Characteristics | Modification sites (cysteine, lysine, glycosylation), spatial conformation, immunogenicity | Cysteine-specific linkers (maleimide, SMCC), lysine-specific linkers | Linkers must be chemically compatible with the antibody to ensure conjugation efficiency and stability while avoiding structural interference or aggregation. |
| In Vivo Release Mechanism | Target cell microenvironment (pH, enzyme activity, reductive environment) | Enzyme-sensitive peptide linkers, acid-sensitive hydrazone linkers, reduction-sensitive disulfide linkers | Enables precise drug release in tumor cells while maintaining stability in healthy tissues, maximizing therapeutic index. |
| Overall Optimization | Stability, DAR uniformity, efficacy, safety | Customized based on specific payload–antibody combination | Multi-parameter optimization improves ADC success rate and reduces clinical development risk. |
| Linker Type | Description |
| Acid Cleavable Linkers | Provide expertise in designing and optimizing acid-sensitive linkers to ensure controlled payload release in acidic tumor microenvironments. |
| Disulfide Linkers | Support the development and modification of disulfide linkers to achieve precise intracellular drug release while maintaining plasma stability. |
| Cathepsin B Cleavable Linkers | Enable the design and synthesis of linkers responsive to Cathepsin B, facilitating efficient enzymatic cleavage within target cells. |
| Phosphatase Cleavable Linkers | Offer capabilities in constructing phosphatase-sensitive linkers for controlled payload activation in specific cellular conditions. |
| Sulfatase Cleavable Linkers | Assist in developing sulfatase-cleavable linkers to optimize drug release in sulfatase-rich environments. |
| β-Galactosidase Cleavable Linkers | Provide support for the creation and optimization of β-galactosidase-sensitive linkers, ensuring selective payload release. |
| β-Glucuronidases Cleavable Linkers | Deliver solutions for designing β-glucuronidase-responsive linkers to achieve targeted and controlled drug delivery. |
Cleavable linkers can be classified into several categories based on their chemical cleavage mechanisms, each offering unique advantages in drug release, targeting specificity, and in vivo stability. Proper selection of a cleavable linker enables efficient drug release within tumor cells while minimizing off-target toxicity, thereby enhancing the ADC's therapeutic index. The main cleavable linker categories and their characteristics are outlined below:
ADC disulfide linkers are cleavable linkers triggered by reducing environments. Within tumor cells, high concentrations of reductants such as glutathione break the disulfide bond, releasing the active drug for rapid intracellular activity. Advantages include:
Development considerations: Disulfide linkers must avoid excessive sensitivity to prevent premature release in blood; the choice of cysteine modification sites also impacts DAR and ADC homogeneity.
ADC hydrazone linkers are pH-sensitive cleavable linkers that primarily cleave in acidic environments, such as lysosomes or endosomes, to release the drug. Characteristics include:
Development considerations: Hydrazone linkers are sensitive to pH fluctuations; their structure must be optimized for the target cell environment to avoid premature drug release in blood or non-target cells.
ADC peptide linkers achieve precise drug release through recognition and cleavage by endogenous enzymes, such as Cathepsin B or Cathepsin L. Advantages include:
Development considerations: Ensure sufficient enzyme activity in target tissues and avoid non-specific degradation in blood or non-target tissues.
ADC glucuronide linkers are cleaved by β-glucuronidase within tumor cells, enabling selective drug release. Features include:
Development considerations: Evaluate variability in tumor enzyme activity and inter-patient differences to ensure effective in vivo drug release.
ADC phosphate linkers are hydrolyzed by specific intracellular phosphatases to release the active drug. Advantages include:
Development considerations: Confirm sufficient phosphatase activity in target cells and consider the drug's sensitivity to enzymatic cleavage to prevent inefficient or excessive release.
Non-cleavable linkers release drugs following antibody degradation, designed to provide highly stable ADCs in circulation, thereby minimizing non-specific drug release and off-target toxicity. While drug release is slower, these linkers significantly enhance in vivo stability and safety, suitable for long-term drug delivery or ADCs requiring high blood stability.
Maleimide linkers are the most widely used non-cleavable linkers, forming stable thioether bonds with cysteine residues on antibodies. Features include:
Development considerations: Optimize cysteine modification sites to avoid disrupting antibody structure and immune activity, and maintain DAR uniformity.
SMCC (Succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate) linkers are non-cleavable heterobifunctional linkers forming stable bonds with antibody amines and cysteines. Advantages include:
Development considerations: Ensure linker structure does not interfere with antibody activity or cause drug aggregation, and control DAR distribution.
SPDB (N-succinimidyl 4-(2-pyridylthio)butanoate) linkers have a stable disulfide structure, releasing drugs gradually following antibody degradation. Features include:
Development considerations: Assess antibody degradation rate in target tissues to ensure effective intracellular drug release.
Functionalized and specialized linkers are highly customizable, optimized for chemical structure, physical properties, and modification strategies to meet complex ADC development requirements for controlled drug release, antibody modification, and in vivo pharmacokinetics. These linkers enhance circulation stability, improve targeted drug release, and provide flexibility for various drug types and antibody formats. Their design is personalized based on drug properties, antibody modification needs, and target tissue environment to achieve efficient and safe ADC efficacy.
ADC PEGylated linkers introduce polyethylene glycol chains into the ADC structure, offering:
Development considerations: PEG length and modification site must be precisely controlled to avoid affecting antibody binding or DAR uniformity; overly long PEG chains may cause steric hindrance.
Amino acid linkers use specific peptide sequences for enzyme-triggered drug release and support diverse antibody modification sites. Advantages include:
Development considerations: Peptides must remain stable in circulation and ensure sufficient target-cell enzyme activity; balance enzyme specificity, linker stability, and antibody compatibility to avoid non-specific or insufficient release.
Selecting an ADC linker should follow a systematic approach, considering drug chemistry, antibody structure, in vivo stability, and pharmacokinetics. A rational linker selection strategy improves efficacy, reduces off-target toxicity, and optimizes therapeutic index. Design should consider four key dimensions: drug-antibody matching, blood stability, DAR optimization, and off-target risk control.
The chemical and biological properties of the drug dictate linker requirements. Lipophilic drugs prone to aggregation or precipitation benefit from PEGylated or non-cleavable linkers to improve solubility and circulation stability. Hydrophilic drugs are better suited for enzyme-sensitive peptide, phosphate, or cleavable hydrazone linkers, which release the drug efficiently in target cells and optimize pharmacokinetics. Drugs requiring rapid intracellular action should use cleavable linkers, such as disulfide or peptide linkers, to ensure fast release and minimize effects on healthy tissue.
Blood stability is a critical design parameter; linkers must prevent premature degradation or drug release, avoiding systemic toxicity and reduced targeting. DAR uniformity is equally important. Controlling the number of drug molecules per antibody (typically 2–8) ensures sufficient efficacy while preventing aggregation, accelerated clearance, or off-target toxicity. Optimizing linker chemistry, conjugation sites, and modification strategies achieves uniform DAR and predictable ADC performance, ensuring intended drug release and distribution.
Off-target toxicity is a major challenge in ADC development. Selecting the appropriate linker type is crucial: cleavable linkers enable rapid targeted release, while non-cleavable linkers provide high blood stability. Optimizing drug release rates through linker sensitivity or functional modifications ensures precise release in target cells while maintaining stability in healthy tissues. Functional modifications, such as PEGylation, peptide design, or amino acid linker optimization, improve solubility, extend circulation, and reduce immunogenicity, collectively enhancing therapeutic index and supporting clinical translation and commercialization.
With years of ADC development and manufacturing experience, BOC Sciences provides end-to-end support from design and optimization to scale-up production. We help clients address complex linker development challenges while optimizing drug release, antibody conjugation, and in vivo stability, accelerating ADC development and increasing preclinical and commercial success rates. From early lab validation to GMP-scale production, BOC Sciences delivers scientific, systematic, and efficient solutions.
From cytotoxins to linkers, explore our cutting-edge products for your ADC project.
| Catalog | Name | CAS | Price |
| BADC-01192 | Sulfo-SMCC sodium | 92921-24-9 | Bulk Inquiry |
| BADC-00894 | SPDB | 115088-06-7 | Bulk Inquiry |
| BADC-00499 | Mal-PEG6-NHS ester | 1599472-25-9 | Bulk Inquiry |
| BADC-00501 | Mc-Val-Cit-PABC-PNP | 159857-81-5 | Bulk Inquiry |
| BADC-00708 | Val-cit-PAB-OH | 159857-79-1 | Bulk Inquiry |
| BADC-01492 | SPDB linker | 1284250-78-7 | Bulk Inquiry |
| BADC-01493 | Sulfo-SPDB linker | 2095682-79-2 | Bulk Inquiry |
| BADC-01514 | Biotin-PEG1-NH2 | 811442-85-0 | Bulk Inquiry |
| BADC-01665 | Sulfo-SPP sodium | 452072-24-1 | Bulk Inquiry |
| BADC-01482 | Fmoc-Val-Ala-OH | 150114-97-9 | Bulk Inquiry |
Explore our advanced tools and expertise for next-generation ADC research and development.
Potent cytotoxins designed for targeted antibody-drug conjugate therapy.
Stable, selective linkers enabling precise drug release in ADCs.
Integrated payload-linker solutions for efficient and targeted ADC delivery.
Targeted therapeutics combining antibodies with cytotoxic drugs for precision treatment.










