Antibody-drug conjugates (ADCs), as a pivotal tool for precision oncology, combine the high specificity of monoclonal antibodies with the potent cytotoxic activity of drugs. However, the overall performance of an ADC does not solely depend on the antibody or the drug; its core component—the linker—plays an equally crucial role in determining the ADC's efficacy, safety, and pharmacokinetic profile. ADC Linkers serve as the chemical bridge between the antibody and the cytotoxic payload, directly influencing the stability of the drug in circulation, the efficiency of intracellular release, and the biodistribution of the entire ADC. Well-designed linkers can ensure the stability of the drug while enabling precise targeted release, thereby maximizing therapeutic outcomes and minimizing off-target toxicity.
Optimizing linker synthesis strategies is an indispensable step in ADC development. This process involves not only selecting suitable chemical reaction types and functional groups but also considering product purity, yield, reproducibility, and scalability. A scientifically sound synthesis design can significantly enhance the overall performance of an ADC while reducing development risks and production costs.
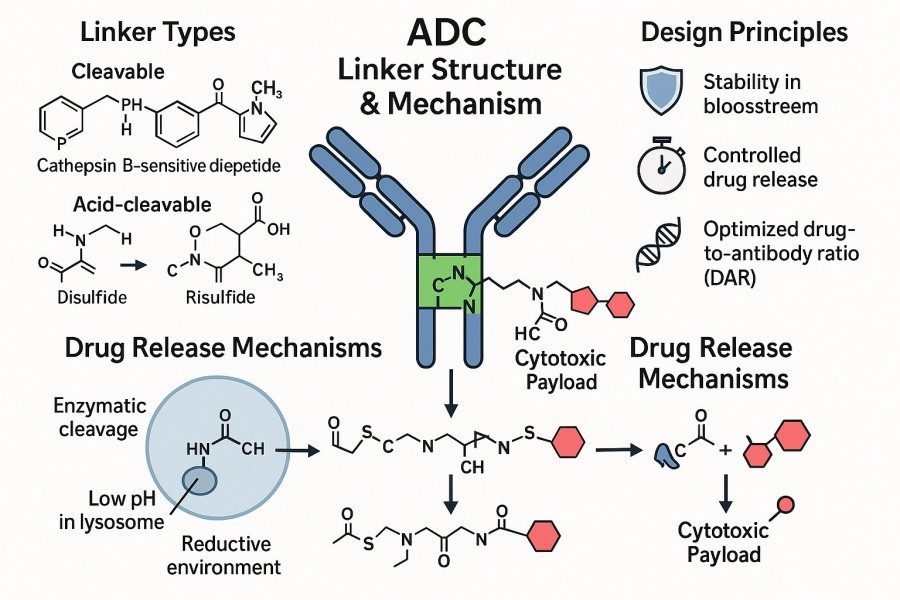 Fig. 1. ADC linker synthesis (BOC Sciences Authorized).
Fig. 1. ADC linker synthesis (BOC Sciences Authorized).
Linker synthesis holds a central position in ADC production. On one hand, the chemical structure of the linker directly determines the stability and circulation time of the ADC in vivo. If the linker is unstable in the bloodstream, premature drug release may occur, reducing therapeutic efficacy and increasing systemic toxicity. On the other hand, the linker also governs the efficiency of payload release within tumor cells. A highly effective and controllable release mechanism ensures that drug concentrations at the target site are sufficient for precise tumor cell killing. Moreover, a high-quality linker synthesis process is crucial for reproducibility and scalability. Laboratory-scale success in linker synthesis, if not scalable, cannot meet the demands of clinical trials or commercial production. Therefore, a scientifically controlled synthesis strategy is essential to bridge ADC development from the laboratory to clinical and market applications.
The chemical properties of linkers play a decisive role in regulating ADC stability and targeted drug release. Cleavable linkers are designed to release the payload under specific stimuli, such as enzymes, pH changes, or reducing environments, ensuring that the drug is released primarily within the tumor microenvironment while sparing healthy tissues. Non-cleavable linkers rely on antibody degradation for drug release and generally exhibit higher stability, prolonging the drug's half-life in circulation. By judiciously selecting linker types and chemical structures, scientists can precisely control ADC stability in blood, tissue distribution, and intracellular drug concentrations. This optimization enhances efficacy while minimizing off-target toxicity, providing a safer and more reliable solution for clinical applications.
Linker design is not only a scientific consideration but also directly affects regulatory compliance and manufacturing feasibility. Regulatory authorities often focus on linker stability, purity, traceability, and impurity control. Complex or unstable linkers may delay approval or limit industrial production. For large-scale manufacturing, the linker synthesis method must be highly reproducible and scalable. Through scientific reaction design, strict quality control, and comprehensive analytical characterization, smooth translation from laboratory synthesis to clinical and commercial production can be ensured. This approach also provides reliable data to support ADC development teams, guaranteeing product consistency and safety throughout clinical and market use.
Choosing the appropriate linker type is critical in ADC design, as it directly impacts drug stability, targeted release capability, and overall therapeutic outcomes. Different linker types have distinct synthetic requirements, and scientists must select the most suitable type based on the drug's properties, target characteristics, and clinical needs.
Cleavable linkers are among the most commonly employed ADC strategies, characterized by their ability to release the payload under specific stimuli. These linkers include enzyme-sensitive, pH-sensitive, and redox-sensitive types:
These cleavable linkers require precise chemical control during design and synthesis to ensure stability and controlled release, ultimately achieving targeted therapy.
Non-cleavable linkers covalently attach the drug to the antibody via highly stable bonds, with drug release primarily depending on antibody degradation in vivo. Commonly using stable amide or maleimide linkages, they feature:
Non-cleavable linkers offer high stability and low off-target toxicity risk, suitable for ADC designs requiring prolonged circulation or antibody-dependent drug release.
The functionality of linkers is central to their design:
Selecting between bifunctional and multifunctional linkers requires balancing ADC design goals, synthetic feasibility, and in vivo performance.
Incorporating spacers is an important strategy to optimize ADC performance. Spacers can:
During synthesis, careful selection and precise introduction of spacers significantly affect final ADC performance, requiring highly controllable chemistry to avoid side reactions or heterogeneous products.
Explore our wide range of high-quality ADC linker products, including cleavable, non-cleavable, stimuli-responsive, and multifunctional options. Tailored for stability, precise payload release, and research-ready applications.
ADC linker synthesis relies on various core chemical reactions, each impacting ADC stability, drug release efficiency, and in vivo behavior. Understanding these reactions helps optimize linker design and ensures controlled, reproducible synthesis suitable for scale-up. Core chemistries include amide bond formation, thiol-maleimide coupling, click chemistry, and carbamate or hydrazone linkages.
Amide bond formation is one of the most commonly used reactions in linker synthesis, valued for its chemical stability and mild reaction conditions. It is widely applied in non-cleavable and some cleavable linkers. By reacting carboxyl and amino groups to form amide bonds, the linker firmly attaches the antibody to the drug while resisting enzymatic degradation in circulation. Activation reagents such as EDC, HATU, or DIC are typically used to facilitate amide bond formation while minimizing byproduct formation. Selectivity and site specificity are critical for ADC homogeneity. Using protective groups and controlled reaction sequences can improve yield and purity, ensuring stable and reliable linker performance.
Thiol-maleimide coupling is an efficient and highly selective covalent conjugation method, often targeting cysteine residues. It proceeds under mild conditions with high yield, making it widely used in ADC linker synthesis. However, it carries potential risks of hydrolysis and side addition reactions. Linker design must control pH, solvent environment, and reaction time to prevent maleimide ring instability, non-specific conjugation, or payload loss. Protective strategies and spacer design can further enhance reaction efficiency and overall ADC stability.
Click chemistry, particularly azide–alkyne cycloaddition, is valued for its high selectivity, mild conditions, and high yield, making it suitable for complex linker synthesis. It enables rapid covalent bond formation without interfering with other functional groups, ideal for multifunctional and cleavable linkers. Click chemistry allows precise incorporation of functional groups, such as fluorescent labels, PEG chains, or additional conjugation sites, optimizing in vivo behavior and drug release. The mild reaction conditions help preserve the activity of sensitive drugs and antibodies during synthesis.
Carbamate and hydrazone bonds are commonly used in pH-sensitive or cleavable linkers. These bonds degrade under acidic conditions, triggering payload release in tumor microenvironments or acidic endosomal compartments. Synthesis requires precise control of temperature, solvents, and reaction time to ensure bond integrity and stability, avoiding premature cleavage in neutral or basic conditions. Optimizing structure and spacer selection allows controlled release rates, maintaining ADC stability in circulation while efficiently releasing drugs inside target cells.
High-quality ADC linker synthesis relies not only on the choice of chemical reactions but also on a scientifically planned synthetic workflow. A systematic workflow ensures that linkers possess stability, purity, and controllable conjugation performance while enhancing scalability from laboratory to industrial production. This workflow typically includes raw material selection, functional group protection and deprotection, bifunctional linker assembly and intermediate purification, and process optimization for scale-up.
The first step in linker synthesis is selecting high-quality raw materials. The purity, structural stability, and traceability of starting materials directly affect the final linker's performance. Impure or unstable chemicals may cause side reactions, low yields, or byproduct formation, ultimately impacting ADC safety and efficacy. Therefore, stringent quality control is required before synthesis, including analysis by nuclear magnetic resonance (NMR), mass spectrometry (MS), and high-performance liquid chromatography (HPLC) to ensure that materials meet high purity and chemical specification standards.
During multi-step synthesis, sensitive functional groups may be affected by non-target reactions, leading to byproducts or reduced yields. Protection strategies are commonly employed to address this issue. Protecting sensitive functional groups prevents nonspecific reactions in key steps, and deprotection restores their activity after specific chemical reactions are completed. For example, in thiol-maleimide coupling, protecting cysteine side chains can prevent oxidation or nonspecific conjugation. Thoughtful design of protection and deprotection steps significantly improves process control and overall yield.
Bifunctional linkers, the most commonly used type in ADC design, require precise control over reaction conditions, purification, and intermediate handling during assembly. Reaction temperature, pH, solvent, and concentration must be carefully controlled to ensure the formation of the desired bonds. Following assembly, intermediates are purified using column chromatography, crystallization, or other efficient methods to remove byproducts and unreacted intermediates. Rigorous purification not only increases the purity of the final product but also ensures controllable drug loading and stability during ADC conjugation.
Scaling up synthesis from laboratory (milligram) levels to gram or industrial production introduces multiple challenges, including:
Through scientific process optimization and process analytics, these scale-up challenges can be effectively addressed, ensuring linker stability and consistency from preclinical to clinical and commercial production.
High-quality ADC linkers require not only precise design and synthesis but also comprehensive analytical characterization to confirm structural accuracy, high purity, and stable performance. Analytical characterization is a critical step in linker development and manufacturing, directly impacting ADC safety, efficacy, and regulatory compliance. Common analytical techniques include nuclear magnetic resonance (NMR), mass spectrometry (MS), high-performance or ultra-performance liquid chromatography (HPLC/UPLC), and stability testing.
Nuclear magnetic resonance (NMR) is a core tool for confirming linker chemical structure and functional group integrity. One-dimensional and two-dimensional NMR analyses can precisely identify amide bonds, thiol-maleimide linkages, hydrazone bonds, and other critical attachment sites. NMR also detects potential byproducts or isomers, ensuring structural uniformity. For complex multifunctional linkers, two-dimensional NMR techniques such as COSY, HSQC, and HMBC are particularly valuable, providing detailed information on atomic connectivity within the molecule.
Mass spectrometry is used to accurately determine the molecular weight and composition of linkers and is a key tool for assessing synthetic success. High-resolution MS (HRMS) or electrospray ionization (ESI-MS) enables rapid detection of the target molecule and byproducts, identifying any mass discrepancies. Additionally, MS can evaluate the degree of linker modification, ensuring controllable conjugation sites and drug loading.
High-performance liquid chromatography (HPLC) or ultra-performance liquid chromatography (UPLC) is the standard method for assessing linker purity and monitoring impurity levels. Optimized chromatographic conditions effectively separate the target product from unreacted intermediates and byproducts, allowing quantitative analysis. Achieving high purity (typically >95%) is a prerequisite for subsequent ADC conjugation and clinical development. HPLC/UPLC also serves as a tool for reaction monitoring and process optimization, ensuring consistent quality across batches.
Linker stability testing evaluates chemical and physical stability under different environmental conditions. Common tests include storage stability at various pH levels (acidic, neutral, basic), accelerated aging studies, and degradation behavior in simulated body fluids or buffer solutions. Stability testing helps predict ADC performance in circulation and within the tumor microenvironment, guiding the selection and optimization of cleavable or non-cleavable linkers to ensure efficient payload release at the target site without premature degradation.
ADC linker synthesis involves multi-step chemical reactions and complex molecular design, presenting multiple technical challenges during both research and production. Overcoming these challenges is critical for ensuring ADC stability, targeting specificity, and safety. Key challenges include the hydrolytic instability of labile bonds, off-target toxicity risks, low yields and impurity formation, and issues related to scale-up and reproducibility.
Many cleavable linkers rely on acid-sensitive, ester, or hydrazone bonds to release the payload. These bonds can undergo hydrolysis during synthesis, storage, or in vitro handling, potentially causing premature drug release or product degradation.
Solutions:
Off-target toxicity mainly arises from uncontrolled drug release in the bloodstream or healthy tissues. Poorly designed cleavable linkers may release the payload prematurely, increasing systemic toxicity.
Solutions:
Multi-step syntheses and complex chemical reactions often lead to reduced yields and impurity formation, especially in bifunctional or multifunctional linker synthesis. Byproducts can interfere with subsequent conjugation steps and compromise ADC structural uniformity.
Solutions:
Linker syntheses successful at laboratory scale may encounter challenges when scaled to gram or industrial production, including uneven reactions, heat generation, insufficient mixing, and batch-to-batch variability. Poor reproducibility directly affects ADC clinical development and commercial production.
Solutions:
Optimizing linker synthesis is essential for improving ADC efficacy and safety. By selecting appropriate chemical moieties, controlling linker length and hydrophilicity, and enhancing conjugation efficiency, ADC stability, targeting specificity, and pharmacokinetic profiles can be significantly improved. Key optimization strategies include:
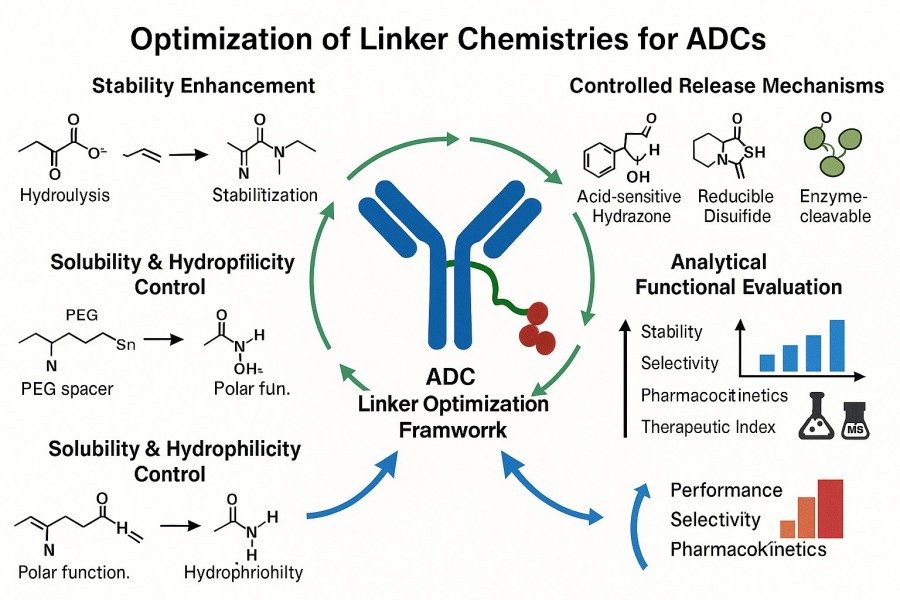 Fig. 2. Optimization of linker chemistries for ADCs (BOC Sciences Authorized).
Fig. 2. Optimization of linker chemistries for ADCs (BOC Sciences Authorized).
Choosing suitable chemical moieties is central to optimizing linker performance. Different functional groups not only affect linker stability but also determine drug release mechanisms and conjugation site selectivity.
Scientific selection of chemical moieties allows for stable, efficient, and controllable targeted drug release.
Linker length and hydrophilicity directly affect ADC spatial conformation, antibody binding, and payload release efficiency.
Precise control of length and hydrophilicity balances ADC stability, targeting, and payload release for optimal therapeutic outcomes.
Conjugation efficiency is a critical performance metric for ADCs, directly affecting drug loading and efficacy. Optimization strategies include:
Enhanced conjugation efficiency ensures linkers provide stable, controllable drug release while supporting high-yield ADC production for clinical and commercial needs.
BOC Sciences offers comprehensive ADC linker synthesis services, from custom design to scalable production, catering to research, clinical development, and commercial applications. Our services cover linker design, modification and optimization, analytical characterization, and cGMP-grade manufacturing, ensuring scientific control and high-quality standards at every step. We design optimal chemical linkage strategies—including cleavable, non-cleavable, bifunctional, or multifunctional linkers—based on ADC structure, drug properties, and clinical requirements. To enhance ADC performance, we also provide linker optimization and modification services, including structural adjustments, spacer introduction, and hydrophobicity tuning.
From cytotoxins to linkers, explore our cutting-edge products for your ADC project.
| Catalog | Name | CAS | Price |
| BADC-01163 | DTSSP Crosslinker | 81069-02-5 | Bulk Inquiry |
| BADC-00417 | endo-BCN-PEG3-NHS ester | 2101206-94-2 | Bulk Inquiry |
| BADC-00439 | MC-PEG2-C2-NHS ester | 1263044-56-9 | Bulk Inquiry |
| BADC-00430 | Propargyl-PEG4-NHS ester | 1428629-70-2 | Bulk Inquiry |
| BADC-00379 | Bis-PEG4-NHS ester | 1314378-11-4 | Bulk Inquiry |
| BADC-00381 | Bis-PEG2-NHS Ester | 65869-63-8 | Bulk Inquiry |
| BADC-01624 | 6-Maleimidocaproic acid | 55750-53-3 | Bulk Inquiry |
| BADC-01159 | Amino-PEG3-propionic acid | 784105-33-5 | Bulk Inquiry |
| BADC-01053 | Propargyl-PEG7-NHS ester | 2093152-77-1 | Bulk Inquiry |
| BADC-01108 | MC-Gly-Gly-Phe-Gly | 2413428-36-9 | Bulk Inquiry |
Explore our advanced tools and expertise for next-generation ADC research and development.
Potent cytotoxins designed for targeted antibody-drug conjugate therapy.
Stable, selective linkers enabling precise drug release in ADCs.
Integrated payload-linker solutions for efficient and targeted ADC delivery.
Targeted therapeutics combining antibodies with cytotoxic drugs for precision treatment.










