In the field of antibody-drug conjugates (ADCs), although the antibody and the cytotoxic payload often receive considerable attention, the linker connecting the two actually plays a critical role in the overall structure and function of an ADC. A well-designed linker not only affects the stability of the ADC in plasma but also determines the timing, location, and rate of drug release, thereby directly influencing the therapeutic index, targeting specificity, safety, and efficacy.
A typical ADC structure consists of three components: a monoclonal antibody (mAb) that specifically binds to tumor antigens, a linker positioned in the middle, and a highly potent cytotoxic payload at the other end. The linker serves as the critical chemical bridge connecting the antibody and the payload.
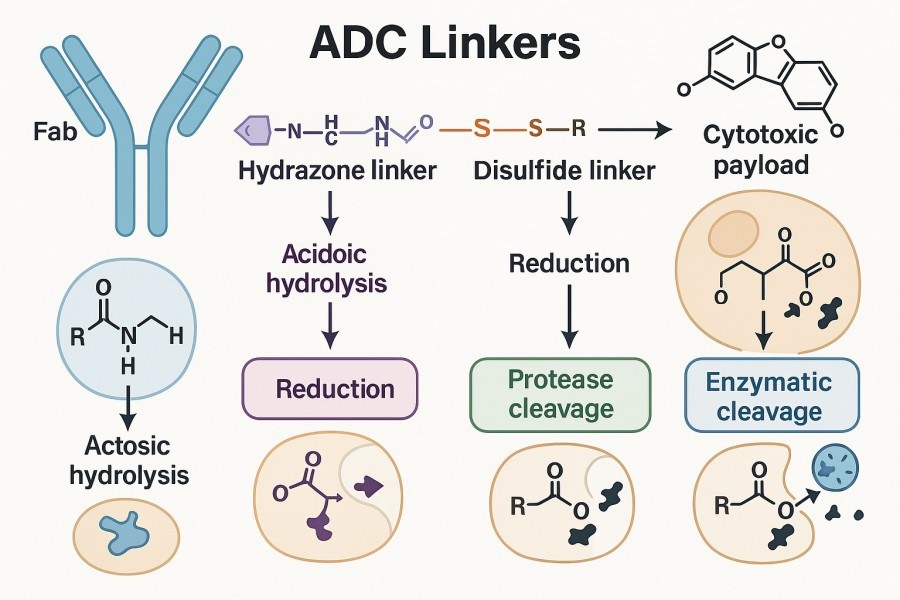 Fig. 1. How the ADC linker works (BOC Sciences Authorized).
Fig. 1. How the ADC linker works (BOC Sciences Authorized).
The linker acts as a "bridge," which must be stable enough in vivo to prevent premature drug release, yet able to trigger payload release timely within target cells or their microenvironment. For example, the first approved ADC, Gemtuzumab ozogamicin (Mylotarg), used an acid-sensitive hydrazone linker. It suffered severe safety issues because the linker degraded too easily in plasma, causing early payload release. Therefore, understanding linker mechanisms is not only a chemical design consideration but also a critical factor for ADC success.
Several major challenges are critical when designing ADC linkers:
Therefore, linker selection and optimization are integral throughout the ADC development process, from early candidate screening and process development to analytical verification and clinical safety/efficacy optimization.
ADC linkers can be broadly classified into cleavable and non-cleavable types based on their cleavage mechanism. The type of linker determines the spatial and temporal characteristics of payload release. Understanding these mechanisms helps in scientifically selecting the appropriate linker to match specific payloads and target requirements.
Cleavable linkers generally operate via:
Non-cleavable linkers operate more passively:
From the perspective of ADC development, the choice between cleavable and non-cleavable linkers can be compared across the following dimensions:
| Feature | Cleavable | Non-Cleavable |
| Plasma Stability | Harder to fully guarantee; risk of premature cleavage | Generally higher stability |
| Payload Release Rate | Faster, allows controlled release | Slower, depends on internalization and degradation |
| Off-Target Toxicity Risk | Higher risk due to premature release | Lower risk of off-target release |
| Support for Bystander Effect | Stronger (released free payload can diffuse) | Weaker (payload may remain inside the cell after release) |
| Suitable Antigen Characteristics | Preferable when antigen internalization is slow or heterogeneous | Better when antigen is highly internalized and uniformly expressed |
| Development Complexity | Requires precise control of cleavage rate | Simpler, but internalization mechanism must be ensured |
Explore our wide range of high-quality ADC linker products, including cleavable, non-cleavable, stimuli-responsive, and multifunctional options. Tailored for stability, precise payload release, and research-ready applications.
The cleavage mechanism of a linker is a critical aspect of ADC design, determining when, where, and how the drug is released inside target cells. Understanding the cleavage behavior of different types of linkers helps optimize ADC stability, selectivity, and therapeutic efficacy. The following sections introduce several typical linker cleavage mechanisms and their key applications.
Acid-sensitive linkers, such as hydrazone and cis-aconityl, were among the earliest types applied in ADC development. Their main feature is sensitivity to acidic environments: they remain relatively stable in neutral plasma (pH ~7.4) with slow hydrolysis rates, but upon entering the acidic environment of endosomes or lysosomes (pH ~4.5–5.5) inside cells, the acidic conditions trigger hydrolysis, breaking the linker and releasing the payload. These linkers have simple structures and are easy to synthesize, making them suitable for early-stage development. However, their main drawback is insufficient plasma stability, which may cause premature drug release and off-target toxicity. Therefore, when designing acid-sensitive linkers, it is crucial to evaluate plasma stability, payload release profiles, and potential non-specific cleavage risks.
Enzyme-cleavable linkers are currently the most widely used and mainstream ADC linker type. These linkers typically contain specific enzymatic cleavage sites that are recognized and cut by enzymes within tumor cells or lysosomes, such as cathepsin B, cathepsin L, or β-glucuronidase. For example, the classic dipeptide valine–citrulline (vc) linker is specifically recognized and cleaved by cathepsin B, followed by self-immolative spacer-mediated release of the active drug. β-Glucuronide linkers rely on overexpressed β-glucuronidase in tumors to trigger drug release, providing strong tumor specificity. The advantages of enzyme-cleavable linkers include controllable release mechanisms, good plasma stability, and precise matching to intracellular tumor environments. The main limitation is that the target tissue must express the corresponding enzymatic activity.
Redox-sensitive linkers exploit differences in reducing environments inside and outside tumor cells for selective drug release. Their typical structure is a disulfide bond (–S–S–), which remains stable in plasma but is reduced to thiols in the highly reductive intracellular tumor environment (rich in glutathione, GSH, up to 100 times higher than normal cells), breaking the linker and releasing the drug. This mechanism enables precise release based on intracellular environmental differences, enhancing targeting. However, if non-target tissues also have high reductive environments, premature drug release may occur. Additionally, for hydrophobic payloads, cleavage may lead to aggregation or limited penetration.
Self-immolative linkers represent an innovative direction in ADC linker design. These structures contain "trigger points" that, once activated (via hydrolysis, enzymatic cleavage, or reduction), initiate a cascade reaction, leading to self-degradation of the linker and eventual payload release. The advantage of self-immolative mechanisms is more efficient and complete drug release, suitable for complex payload systems. For example, DNA-intercalating agents, PBD drugs, or highly cytotoxic molecules often use multi-step cleavage logic: first enzymatic or chemical triggering, followed by self-immolative degradation to generate the active drug. In next-generation ADC development, self-immolative linkers are widely used to design "smart release systems" responsive to multiple stimuli such as pH, enzymes, redox conditions, electrochemical signals, or metal ions.
The chemical properties of linkers directly determine ADC stability in vivo, payload release rate, and therapeutic outcome. A well-designed linker must remain stable in plasma while achieving efficient cleavage and precise release inside target cells. The following sections explore the profound impact of linker mechanisms on ADC stability and efficacy across three dimensions.
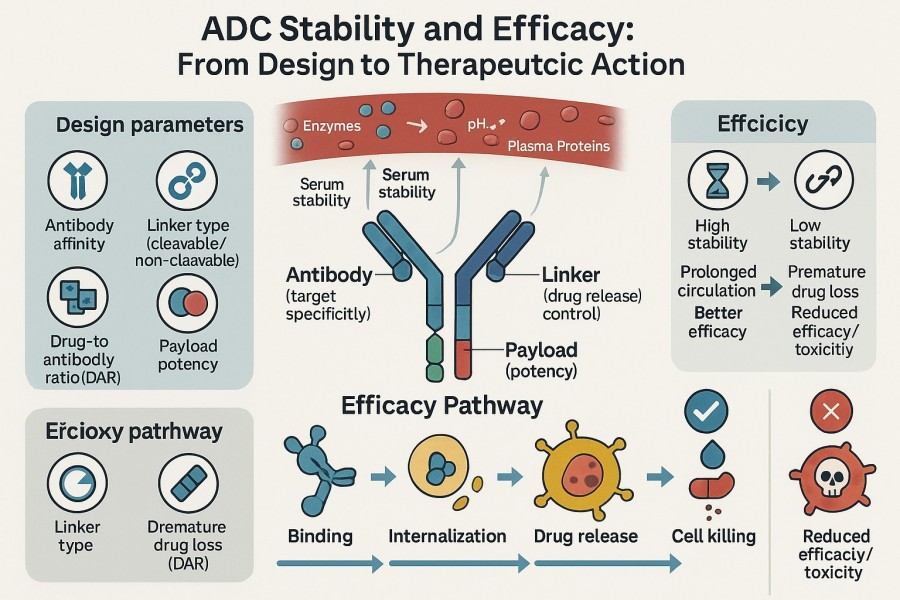 Fig. 2. ADC stability and efficacy (BOC Sciences Authorized).
Fig. 2. ADC stability and efficacy (BOC Sciences Authorized).
Linker stability is a critical prerequisite for ADC success. Premature cleavage in plasma causes early payload release, leading to off-target toxicity, reduced effective drug concentration, and shortened circulation half-life. Conversely, overly stable linkers can hinder payload release in target cells, reducing therapeutic efficacy. In ADC development, designers must balance "stability" and "release rate." Linkers should possess sufficient chemical stability in plasma to prevent non-specific cleavage and antibody aggregation, while efficiently responding to intracellular acidic, enzymatic, or reductive conditions for precise drug release. Common stability evaluation metrics include:
Beyond controlling payload release, linker chemistry profoundly affects ADC physicochemical properties, including solubility, toxicity distribution, antibody aggregation propensity, and targeting specificity.
Linker chemistry not only influences molecular stability but also directly affects ADC pharmacokinetics (PK) and pharmacodynamics (PD), ultimately determining the therapeutic index.
In ADC research and development, validating and characterizing linker mechanisms is a critical step to ensure successful drug performance. The chemical properties of linkers not only determine the stability of the payload in circulation but also influence its release efficiency within target cells. Therefore, establishing systematic analytical methods is essential for evaluating linker behavior, stability, and release kinetics.
Studying linker stability and cleavage rates is central to understanding ADC performance and pharmacokinetics. Common experimental and analytical methods include:
Mass spectrometry (MS) and chromatographic techniques are indispensable for studying ADC linkers, providing critical information on linker structure, cleavage products, and conjugation efficiency. Key analytical approaches include:
As ADC technology matures, linker design is evolving from basic "stability and release" functions toward "precise, intelligent, and controllable" systems. While traditional acid-, enzyme-, or redox-sensitive linkers are widely used in commercial ADCs, the complexity of tumor microenvironments and diverse payload requirements drives the innovation of next-generation linkers. The following strategies highlight cutting-edge trends in ADC linker development.
Stimuli-responsive linkers are a major focus in recent ADC research. These linkers respond to specific physiological triggers (e.g., low pH, enzyme activity, high levels of reactive oxygen species (ROS), or light) to achieve precise cleavage and payload release within target tissues or cells. For example, light-responsive linkers enable spatiotemporally controlled release via external light signals, greatly enhancing delivery precision. ROS-responsive designs exploit the high oxidative stress in tumor tissues to selectively trigger cleavage at the disease site. Such strategies significantly improve ADC selectivity and safety.
Dual-cleavable and multi-functional linkers represent another innovative ADC design approach. These structures integrate two or more cleavage mechanisms, such as enzymatic plus redox-triggered, or enzymatic combined with light-triggered, to achieve higher release precision in complex or heterogeneous tumor microenvironments. Some designs also incorporate self-immolative and targeting modules, such as PEG shielding for improved solubility or specific ligands to enhance cellular uptake, further optimizing in vivo behavior. These multifunctional structures enhance controllable payload release and provide a structural foundation for next-generation high-efficiency, low-toxicity ADCs.
Introducing functional modifications, such as PEGylation or biotinylation, is becoming an important strategy to enhance ADC performance. PEGylated linkers incorporate polyethylene glycol (PEG) segments into the backbone, improving hydrophilicity, reducing aggregation caused by hydrophobic payloads, and enhancing drug stability and circulation time. Biotinylated linkers leverage the strong affinity between biotin and streptavidin to achieve precise targeting, capture, or signal amplification, offering new strategies for ADC detection, imaging, and controlled release. Although these linkers are at an early stage of clinical application, their potential in multifunctional drug design and bioanalysis is widely recognized.
BOC Sciences provides global clients with one-stop technical services covering linker design, custom synthesis, structural optimization, and analytical characterization. By combining advanced chemical synthesis platforms, molecular optimization strategies, and multimodal analytical technologies, we help clients rapidly obtain high-quality linkers while ensuring ADC stability, targeting, and therapeutic index. Whether traditional cleavable linkers or next-generation stimuli-responsive, multifunctional structures, BOC Sciences offers professional custom solutions to accelerate the development and translation of novel ADC therapeutics.
From cytotoxins to linkers, explore our cutting-edge products for your ADC project.
| Catalog | Name | CAS | Price |
| BADC-00364 | Fmoc-Val-Cit-PAB | 159858-22-7 | Bulk Inquiry |
| BADC-00698 | Boc-Val-Cit-PAB-PNP | 870487-10-8 | Bulk Inquiry |
| BADC-00382 | Bis-PEG1-NHS ester | 65869-64-9 | Bulk Inquiry |
| BADC-00982 | Fmoc-N-amido-PEG2-acetic acid | 166108-71-0 | Bulk Inquiry |
| BADC-01586 | Fmoc-Gly-Gly-Gly-Gly-OH | 1001202-16-9 | Bulk Inquiry |
| BADC-00906 | Propargyl-PEG5-acid | 1245823-51-1 | Bulk Inquiry |
| BADC-01633 | 3-Azidopropanol | 72320-38-8 | Bulk Inquiry |
| BADC-01140 | DSP Crosslinker | 57757-57-0 | Bulk Inquiry |
| BADC-00942 | Fmoc-Val-Ala-PAB-OH | 1394238-91-5 | Bulk Inquiry |
| BADC-01482 | Fmoc-Val-Ala-OH | 150114-97-9 | Bulk Inquiry |
Explore our advanced tools and expertise for next-generation ADC research and development.
Potent cytotoxins designed for targeted antibody-drug conjugate therapy.
Stable, selective linkers enabling precise drug release in ADCs.
Integrated payload-linker solutions for efficient and targeted ADC delivery.
Targeted therapeutics combining antibodies with cytotoxic drugs for precision treatment.










