In the development of antibody-drug conjugates (ADCs), the choice of linker is critically important. Linkers not only determine ADC stability and drug release efficiency but also directly impact therapeutic efficacy and safety. Therefore, selecting an ADC linker scientifically and rationally is a key step to improving the success rate of ADC therapeutics. This article provides a systematic analysis of the role of ADC linkers, key selection considerations, common challenges, and optimization strategies, helping research and pharmaceutical teams make informed decisions.
The linker is a core component of ADCs, serving as the bridge that reliably connects a monoclonal antibody (mAb) with a cytotoxic payload. It not only provides structural linkage but also directly influences the transport, distribution, and release efficiency of the drug in vivo. Rational linker design is crucial for achieving precise targeted therapy. Specifically, ADC linkers perform the following functions:
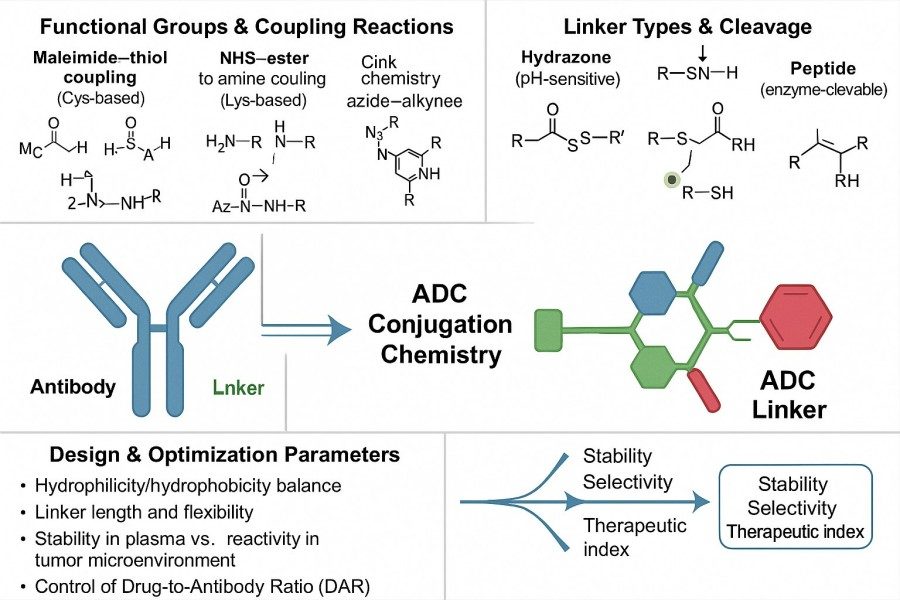 Fig. 1. ADC linker chemistry (BOC Sciences Authorized).
Fig. 1. ADC linker chemistry (BOC Sciences Authorized).
Thus, the linker is not just a structural element of ADCs but a key determinant of their efficacy and safety.
ADC linkers are generally classified into cleavable and non-cleavable types.
Cleavable linkers rely on tumor-specific microenvironment conditions to release the drug, such as pH-sensitive, enzyme-sensitive, or reduction-sensitive linkers. pH-sensitive linkers break in acidic intracellular environments to release the payload; enzyme-sensitive linkers are cleaved in the presence of specific proteases, ensuring that the drug is activated only within target cells. Cleavable linkers enable precise drug release, enhancing targeting specificity and therapeutic outcomes.
Non-cleavable linkers, on the other hand, release the drug through antibody degradation pathways. The payload is released only after the antibody is degraded in the lysosome of target cells, resulting in higher circulatory stability. This type of linker typically reduces the risk of nonspecific drug release in the bloodstream but requires sufficient antibody-payload degradation efficiency to achieve therapeutic effect.
The mechanism of each linker type directly influences ADC behavior in vivo, including drug release rate, circulatory half-life, and tissue distribution. Therefore, selecting the appropriate linker type based on drug properties, tumor target characteristics, and therapeutic strategy is critical in ADC design.
Linkers covalently bind the antibody to the drug while maintaining the integrity of both the antibody structure and drug activity. Common linkage types include thioether bonds, amide bonds, and other functional chemical bonds. When choosing a linkage, multiple factors must be considered: antibody structural stability, drug activity protection, conjugation efficiency, and in vivo release control. For example, thioether bonds provide high blood stability, suitable for reducing nonspecific release, while certain amide or ester bonds can be designed as cleavable linkers to achieve rapid intracellular drug release. Through rational design, linkers can remain stable in circulation while efficiently releasing active drugs within target cells, enabling precise therapy.
ADC Linker design directly impacts ADC efficacy, safety, and pharmacokinetics/pharmacodynamics (PK/PD). Insufficiently stable linkers may cause premature drug release in the bloodstream, leading to off-target toxicity and potential damage to healthy tissues. Conversely, inefficient or poorly timed release may reduce drug concentration in target cells, weakening therapeutic effect. Additionally, linker design affects ADC half-life, tissue distribution, and immunogenicity. For example, cleavable linkers that rapidly release the drug in tumor cells can improve treatment selectivity, while non-cleavable linkers prolong circulation time, reducing systemic toxicity risks. Overall, linkers are not merely chemical bridges but critical variables determining ADC success. Their rational design is essential for achieving efficient and safe therapy.
Explore our wide range of high-quality ADC linker products, including cleavable, non-cleavable, stimuli-responsive, and multifunctional options. Tailored for stability, precise payload release, and research-ready applications.
When selecting an ADC linker, multiple key factors must be evaluated. These factors not only influence ADC circulatory stability and drug release efficiency but also directly affect pharmacokinetics, pharmacodynamics, safety, and clinical development feasibility. A scientific assessment of these factors helps R&D teams design ADCs that are efficient, safe, and have controllable drug release characteristics.
Selecting the appropriate linker in ADC development is not straightforward. Linkers must maintain stability in circulation, release the drug precisely in target cells, and avoid increasing off-target toxicity. Additionally, linkers must be highly compatible with both antibody and payload to ensure conjugation efficiency and overall ADC performance. ADC development teams face multiple challenges in linker design and selection, requiring a careful balance of stability, release efficiency, safety, and compatibility.
A primary challenge in linker design is achieving a balance between circulatory stability and drug release efficiency. Overly stable linkers may delay drug release within target cells, leading to insufficient intracellular accumulation and reduced therapeutic effect. Conversely, unstable linkers may release drugs prematurely in circulation, causing off-target toxicity and side effects. Scientists typically achieve this balance by precisely controlling linker chemistry, conjugation sites, and functional group selection. For example, adjusting the sensitivity of ester or thioether bonds in cleavable linkers can maintain drug stability in circulation while ensuring rapid release in the tumor microenvironment.
Off-target toxicity is a core issue in ADC development, directly affecting patient safety and the therapeutic window. Selecting the appropriate linker type, optimizing drug release mechanisms, and controlling the DAR are key strategies to minimize off-target toxicity. Excessive DAR may increase free drug concentration in the blood, harming healthy tissues. Imprecise release mechanisms may activate the drug in non-target cells, increasing side effects. Therefore, scientists must design linker structures that are both safe and effective by considering drug chemistry, tumor microenvironment characteristics, and antibody properties.
Linker compatibility with both antibody and payload directly determines ADC conjugation efficiency and overall performance. Variations in antibody structure, payload functional groups, and reaction conditions all influence conjugation success and stability. For example, drugs containing multiple thiol or amino groups may require specific maleimide or ester linkers for effective conjugation. Additionally, linker structures must not interfere with antibody folding, stability, or drug activity. Considering these factors ensures ADCs maintain high quality and consistency during development and manufacturing while achieving stable drug release and high therapeutic efficacy.
Although there are many types of linkers, each with distinct characteristics in terms of stability, drug release efficiency, and safety, R&D teams can develop a scientific selection strategy through systematic analysis of the antibody, payload, and target cell properties. The following are key practical tips for selecting ADC linkers.
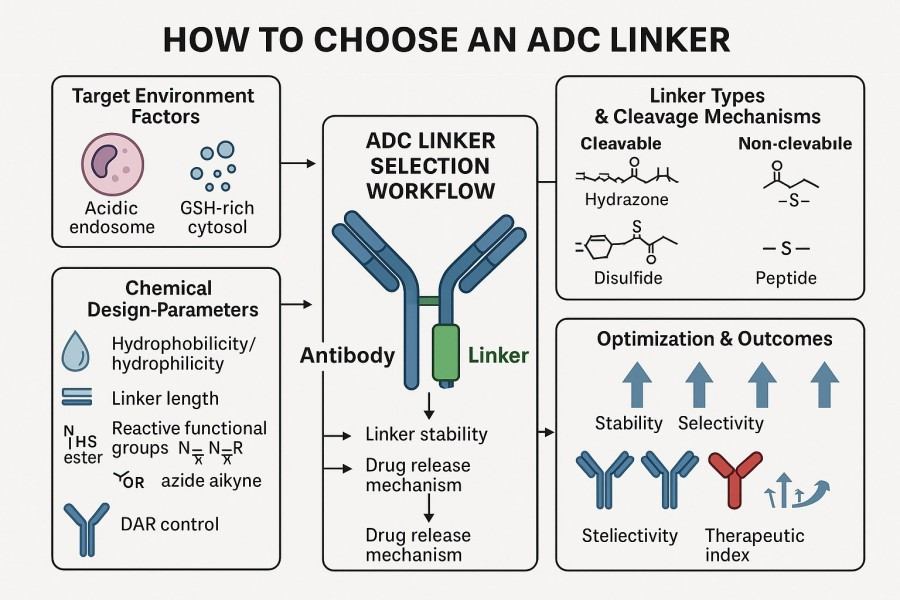 Fig. 2. How to choose an ADC linker (BOC Sciences Authorized).
Fig. 2. How to choose an ADC linker (BOC Sciences Authorized).
Optimizing ADC linker selection is a critical step to ensure the drug is efficient, safe, and exhibits controlled release. Scientific optimization strategies involve not only the chemical design of linkers but also comprehensive evaluation of physicochemical properties, in vitro and in vivo stability testing, and structure-activity relationship (SAR) analysis. A systematic approach can significantly improve the therapeutic index and clinical feasibility of ADCs.
In ADC development, linker selection and optimization is a highly specialized process that directly impacts drug stability, targeting, and therapeutic effect. BOC Sciences has extensive experience in ADC research and advanced technology platforms, offering full-process solutions from linker design, synthesis, and conjugation to analytical evaluation and process optimization. With strong chemical synthesis capabilities, precise conjugation techniques, and rigorous quality control systems, BOC Sciences helps clients accelerate ADC development while ensuring scientific rigor, safety, and feasibility.
From cytotoxins to linkers, explore our cutting-edge products for your ADC project.
| Catalog | Name | CAS | Price |
| BADC-00933 | DBCO-NHS ester | 1353016-71-3 | Bulk Inquiry |
| BADC-00415 | BCN-PEG4-NHS ester | 1702356-19-1 | Bulk Inquiry |
| BADC-00405 | NHS-PEG4-azide | 944251-24-5 | Bulk Inquiry |
| BADC-00374 | m-C-tri(CH2-PEG1-NHS ester) | 173414-89-6 | Bulk Inquiry |
| BADC-01135 | Fmoc-N-amido-PEG4-propionic acid | 557756-85-1 | Bulk Inquiry |
| BADC-01402 | (p-SCN-Bn)-NOTA | 147597-66-8 | Bulk Inquiry |
| BADC-01172 | Azido-PEG5-alcohol | 86770-68-5 | Bulk Inquiry |
| BADC-01944 | Boc-D-trans-Hyp-Ome | 135042-17-0 | Bulk Inquiry |
| BADC-01623 | 3-Maleimidopropionic acid | 7423-55-4 | Bulk Inquiry |
| BADC-00980 | Fmoc-Phe-Lys(Boc)-PAB-PNP | 1646299-50-4 | Bulk Inquiry |
Explore our advanced tools and expertise for next-generation ADC research and development.
Potent cytotoxins designed for targeted antibody-drug conjugate therapy.
Stable, selective linkers enabling precise drug release in ADCs.
Integrated payload-linker solutions for efficient and targeted ADC delivery.
Targeted therapeutics combining antibodies with cytotoxic drugs for precision treatment.










