Antibody-drug conjugates (ADCs), as a breakthrough innovation in recent cancer therapy, combine the targeting specificity of monoclonal antibodies with the potent cytotoxicity of small-molecule drugs, achieving the ideal effect of precise delivery and site-specific release. However, the stability and safety of ADCs largely depend on their critical chemical bridge—the ADC linker. The linker not only determines when and where the payload is released but also directly influences the ADC's toxicological profile, circulation time, and clinical efficacy. If the linker breaks prematurely or degrades in non-target tissues, off-target toxicity occurs, reducing therapeutic efficacy and increasing side effects.
The design philosophy of ADCs follows a "Trojan horse" strategy, using the antibody's targeting specificity to hide and deliver the toxin (small-molecule cytotoxic drug) to tumor sites. As the "bridge" between the antibody and the toxin, the linker plays a pivotal role.
ADCs are innovative biotherapeutics typically composed of three key components: a highly specific monoclonal antibody (mAb), a potent cytotoxic small-molecule drug (payload/cytotoxin), and a chemical linker. The antibody recognizes and binds to specific antigens on tumor cell surfaces; the toxin induces apoptosis inside the cell; and the linker remains stable during circulation, releasing the payload only upon reaching the tumor microenvironment or after cellular internalization. This precise delivery mechanism gives ADCs tremendous potential in clinical applications.
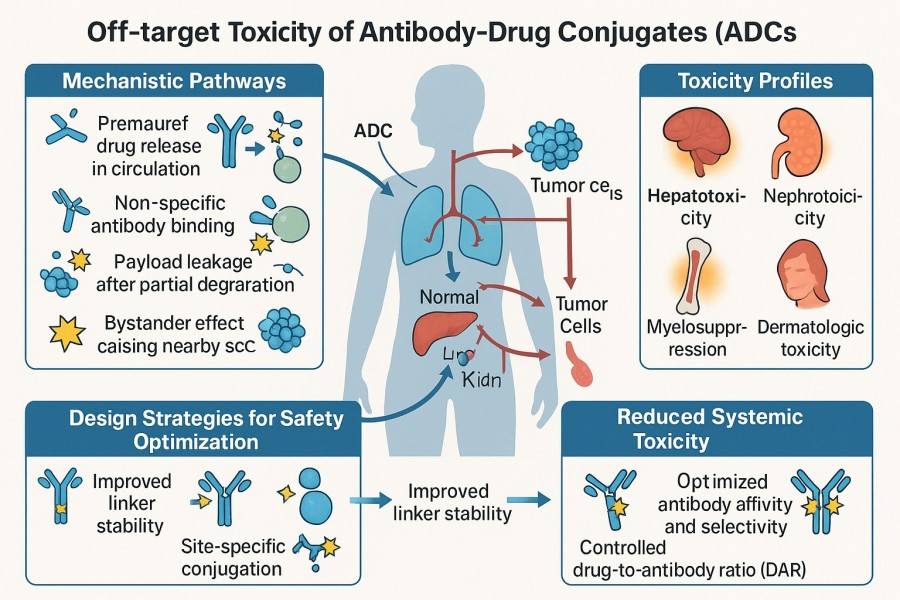 Fig. 1. Off-target toxicity of ADC (BOC Sciences Authorized).
Fig. 1. Off-target toxicity of ADC (BOC Sciences Authorized).
Linker stability refers to the ability of an ADC to maintain its conjugated state in plasma and non-target tissues, preventing payload release until exposure to the specific intracellular environment of target cells triggers cleavage or release. Its importance is reflected in:
Thus, in ADC design, the linker must be "strong enough" to remain intact but also "cleavable on demand"—a delicate balance.
Off-target toxicity is a major challenge limiting ADC safety and effectiveness. It occurs when the ADC or its released payload adversely affects non-target cells or tissues, leading to dose-limiting toxicity (DLT), treatment interruption, or failure. For ADCs, off-target toxicity can arise from multiple mechanisms, including low-level expression of target antigens in normal tissues (on-target/normal tissue toxicity), non-specific antibody binding, premature linker/payload release in circulation, bystander effects, or abnormal enzyme-mediated release. Its consequences include:
Therefore, considering off-target toxicity throughout ADC design, analysis, and manufacturing is essential for improving project success rates.
Designing an ADC linker is a sophisticated chemical engineering task. Its stability is influenced by the linker's chemical structure, the physiological environment, and the conjugation method.
The chemical structure and properties of the linker are primary determinants of stability. Structural design considerations include: cleavable versus non-cleavable linkers; triggering mechanisms (e.g., enzymatic, reductive, acidic); linker length, configuration, steric hindrance, hydrophilicity/hydrophobicity; and the method of attachment to antibody/payload. For example:
Thus, linker design must balance "sufficient stability to prevent off-target release" with "reliable triggerability for therapeutic action."
Beyond the linker's intrinsic structure, the physiological environment—such as plasma proteases, reductants (e.g., GSH), pH, temperature, plasma protein binding, and cellular uptake—significantly affects degradation and cleavage rates.
In summary, linker design aims for "high plasma stability and release triggered by target-specific conditions," accounting for multiple in vivo degradation mechanisms.
The chemistry and site of attachment between the antibody, linker, and payload are also crucial for ADC stability. Conjugation approaches include lysine linkage, inter-chain cysteine linkage, and site-specific cysteine mutations. Studies indicate:
Therefore, linker stability optimization involves not only chemical design but also site selection, DAR control, and conjugation technology—a systemic engineering challenge.
Explore our wide range of high-quality ADC linker products, including cleavable, non-cleavable, stimuli-responsive, and multifunctional options. Tailored for stability, precise payload release, and research-ready applications.
Off-target toxicity is one of the most challenging safety concerns in ADC development. Its mechanisms are complex, involving premature payload release, immune responses, and non-specific binding.
Off-target toxicity often arises from unexpected drug release in non-target tissues or immune activation. Instability of the linker, cross-reactivity of the antibody, and abnormal enzyme activation can all contribute.
Linker stability is closely and inversely related to off-target toxicity. Insufficient stability in circulation leads to premature payload release, exposing normal tissues to cytotoxic components and causing systemic side effects. Excessive stability, however, can hinder payload release in target cells, weakening therapeutic efficacy. Therefore, the key in linker design is balancing stability and cleavability. By optimizing bond types (e.g., amide, disulfide, carbon-nitrogen bonds) and spatial configuration, dual features of high plasma stability and controlled tumor-triggered cleavage can be achieved. Studies show that enzymatically or reduction-sensitive linkers effectively reduce premature plasma release, significantly mitigating off-target toxicity and improving overall ADC safety and efficacy.
Enhancing linker stability is a key step in improving ADC performance and safety. In recent years, researchers have continuously improved plasma stability and target responsiveness of linkers through molecular structure optimization, control of reaction sites, and physicochemical modification strategies. An ideal linker should remain intact in circulation and efficiently cleave in the specific tumor microenvironment (e.g., low pH, reductive conditions, or enzyme-specific triggers), enabling precise drug release with minimal toxic exposure.
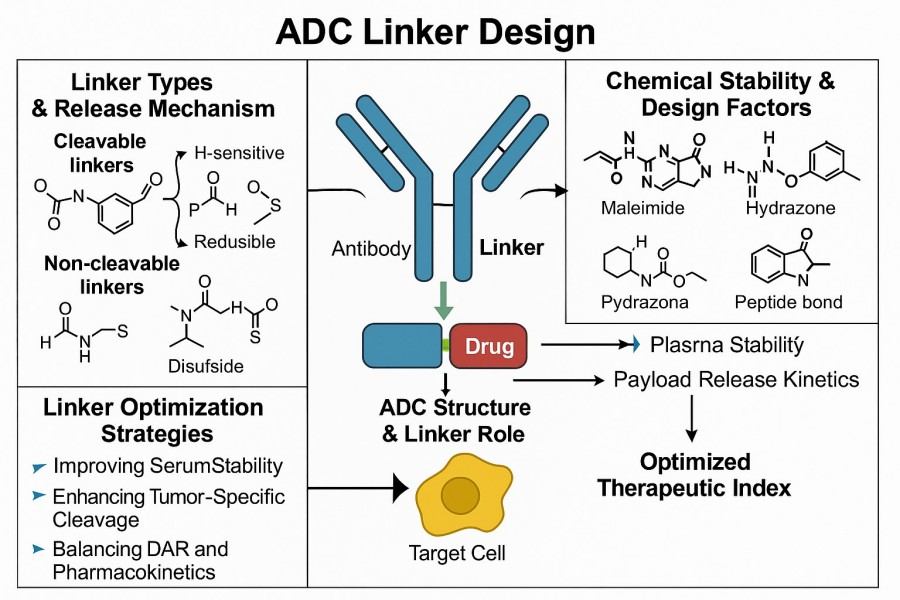 Fig. 2. ADC linker design (BOC Sciences Authorized).
Fig. 2. ADC linker design (BOC Sciences Authorized).
In ADC design, both cleavable and non-cleavable linkers have their advantages. Cleavable linkers (e.g., enzyme-sensitive linker, acid-sensitive linker, or reduction-sensitive linker) can achieve "intelligent release" of the payload by recognizing specific intracellular conditions in tumor cells, significantly enhancing drug selectivity. Non-cleavable linkers rely on lysosomal degradation of the antibody to release the payload, maintaining high stability in circulation and reducing systemic toxicity. To balance stability and efficacy, researchers typically select the appropriate type based on the internalization rate of the target antigen, payload properties, and therapeutic requirements, while adjusting chemical bond strength (e.g., thioether, carbon–nitrogen bonds) to achieve the desired stability. Optimization strategies include:
With the advancement of chemical biology, modern linker design is trending toward "intelligent" and "controllable" strategies, aimed at minimizing off-target toxicity. Specific developments include:
Scientific evaluation of linker stability is an essential step in ADC development. Techniques such as LC-MS/MS, HPLC, mass spectrometry-based quantification, and stability kinetics testing allow precise monitoring of linker cleavage rates and degradation products under in vitro and in vivo simulated conditions. These analyses help identify stability differences of various linker structures in plasma, cell lysates, and physiological buffers, optimizing linker chemistry and reaction site design to ensure batch consistency and predictability. Key methods include:
Beyond traditional cleavable or non-cleavable linkers, research increasingly focuses on controlled release mechanisms, which trigger payload release only within the tumor microenvironment or inside target cells, maximizing targeting while minimizing off-target effects. Design strategies include:
Reducing off-target toxicity is critical to improving clinical safety and the therapeutic window of ADCs. In development, off-target toxicity often arises from premature payload release, non-specific antibody binding, or immunogenic responses. Therefore, systematic integration of strategies across antibody selection, payload optimization, linker chemistry, and analytical evaluation is essential to ensure ADC safety and efficacy.
While linker optimization is critical, antibody specificity remains the primary defense against off-target toxicity. Enhancing antibody affinity and specificity for tumor-specific antigens, minimizing cross-reactivity with normal tissue antigens, and avoiding Fc receptor or other non-specific receptor binding are key considerations. For instance, if the target antigen is expressed at low levels in normal tissues, even a stable linker with precise release can still cause normal cell toxicity. Early-stage ADC development should optimize antibody, linker, and payload synergy to fundamentally control off-target toxicity.
The potency and release characteristics of the payload are critical factors in off-target toxicity. Highly potent payloads with strong diffusibility may exacerbate bystander effects, harming normal tissues. Payload optimization strategies include:
Selecting appropriate linker chemistries—stable, low immunogenicity, and highly biocompatible—is essential to minimize off-target toxicity. Recommended practices include:
Multidimensional analysis provides a scientific basis for minimizing off-target toxicity. Combining in vitro cytotoxicity screening, plasma stability testing, animal model evaluation, and pharmacokinetic analysis allows comprehensive monitoring of ADC drug release and toxicity profiles under various conditions. Recommended approaches include:
In ADC development, linker stability and control of off-target toxicity are key determinants of candidate success. BOC Sciences is committed to providing global clients with comprehensive, customized ADC linker optimization services—from molecular design and chemical synthesis to analytical characterization—helping research teams achieve high stability, high selectivity, and low toxicity in ADC products.
From cytotoxins to linkers, explore our cutting-edge products for your ADC project.
| Catalog | Name | CAS | Price |
| BADC-00933 | DBCO-NHS ester | 1353016-71-3 | Bulk Inquiry |
| BADC-00369 | Fmoc-Val-Cit-PAB-PNP | 863971-53-3 | Bulk Inquiry |
| BADC-00405 | NHS-PEG4-azide | 944251-24-5 | Bulk Inquiry |
| BADC-01147 | DSS Crosslinker | 68528-80-3 | Bulk Inquiry |
| BADC-01192 | Sulfo-SMCC sodium | 92921-24-9 | Bulk Inquiry |
| BADC-00698 | Boc-Val-Cit-PAB-PNP | 870487-10-8 | Bulk Inquiry |
| BADC-00498 | Propargyl-NHS Ester | 1174157-65-3 | Bulk Inquiry |
| BADC-00906 | Propargyl-PEG5-acid | 1245823-51-1 | Bulk Inquiry |
| BADC-00942 | Fmoc-Val-Ala-PAB-OH | 1394238-91-5 | Bulk Inquiry |
| BADC-01482 | Fmoc-Val-Ala-OH | 150114-97-9 | Bulk Inquiry |
Explore our advanced tools and expertise for next-generation ADC research and development.
Potent cytotoxins designed for targeted antibody-drug conjugate therapy.
Stable, selective linkers enabling precise drug release in ADCs.
Integrated payload-linker solutions for efficient and targeted ADC delivery.
Targeted therapeutics combining antibodies with cytotoxic drugs for precision treatment.










