Antibody–drug conjugates (ADCs) have become one of the fastest-growing classes of drugs in modern precision oncology. From early products like Mylotarg to widely marketed ADCs today, such as Enhertu, Padcev, and Zynlonta, linkers and conjugation chemistry have been proven to be core factors that determine ADC efficacy, stability, safety, and commercial success.
In an ADC structure, the linker is responsible for securely and stably connecting the small-molecule drug to the monoclonal antibody, while conjugation chemistry involves the specific chemical methods and reaction strategies used to achieve effective and controlled drug loading. Linkers and conjugation chemistry together define the functional performance of ADCs and their potential for clinical applications.
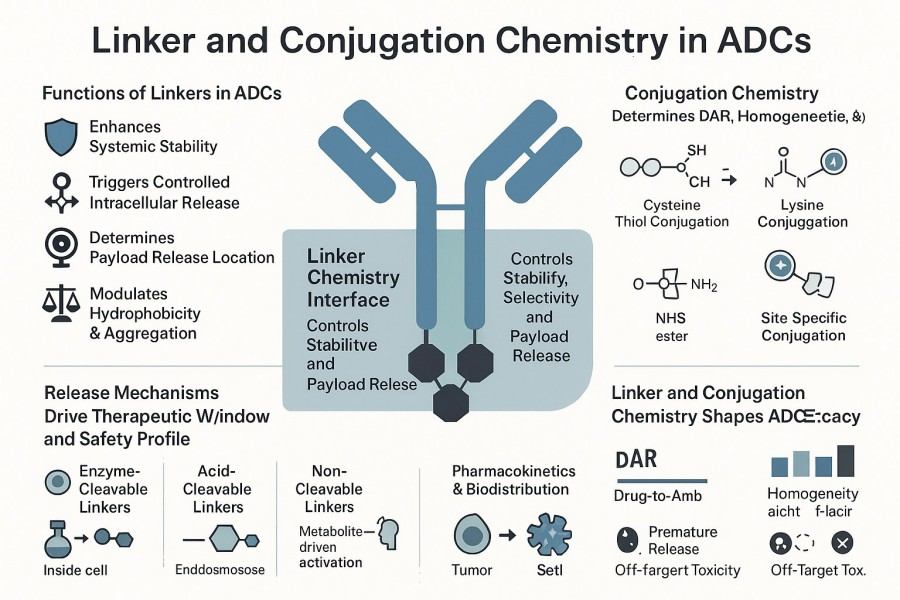 Fig. 1. Linker and conjugation chemistry in ADCs (BOC Sciences Authorized).
Fig. 1. Linker and conjugation chemistry in ADCs (BOC Sciences Authorized).
The choice of linker directly affects ADC stability and selectivity in vivo. An ideal linker should remain stable in the bloodstream to prevent premature drug release while enabling efficient payload release within target cells for therapeutic effect. Cleavable linkers release the payload in response to specific enzymes, acidic conditions, or reducing environments, whereas non-cleavable linkers rely on antibody degradation pathways. The chemical properties, spatial structure, and hydrophobic/hydrophilic modifications of the linker all influence ADC circulation stability and target selectivity.
Conjugation chemistry determines the attachment sites, conjugation efficiency, and distribution uniformity of the payload. Different conjugation approaches can affect the DAR (drug-to-antibody ratio), antibody conformation, and drug release characteristics. For example, lysine conjugation often results in random labeling, whereas cysteine conjugation via partial reduction of antibody disulfides enables controlled labeling. Enzyme-mediated and bioorthogonal conjugation can achieve highly uniform, site-specific attachment, optimizing both drug activity and safety.
The design of linkers and conjugation chemistry directly impacts overall ADC performance, including stability, pharmacokinetics, and targeting efficiency. Proper design can enhance therapeutic efficacy while minimizing off-target toxicity.
The selection and mechanistic design of ADC linkers are central to ADC performance. Linkers influence not only ADC stability in the bloodstream but also how efficiently the drug is released within target cells, directly affecting efficacy, safety, and pharmacokinetic profiles. Based on the release mechanism, ADC linkers are generally classified as cleavable, non-cleavable, or self-immolative spacer systems.
Cleavable linkers are the most commonly used type in ADC development. Their core advantage lies in triggering drug release under specific stimuli, enabling "on-demand" delivery. This allows precise payload release inside target cells, enhancing antitumor activity while reducing systemic toxicity. Cleavable linkers are mainly categorized into three types:
These linkers are cleaved by proteases highly expressed in the tumor microenvironment or lysosomes. Examples include Cathepsin B-sensitive linkers (Val-Cit, Phe-Lys), and linkers targeting Cathepsin L, D, or K. Mechanistic features:
Representative product: Brentuximab vedotin (Adcetris) uses a Val-Cit linker to release MMAE via Cathepsin B cleavage.
These linkers exploit the low-pH environment in tumors or intracellular vesicles to release the payload. Common structures include Hydrazone and Cis-aconityl groups. Mechanistic features:
Representative product: Gemtuzumab ozogamicin (Mylotarg) uses an acid-sensitive hydrazone linker to release calicheamicin.
These linkers release the payload in response to high intracellular glutathione (GSH) or other reducing conditions in tumor cells, commonly featuring disulfide bonds or electronically tuned disulfides. Mechanistic features:
Non-cleavable linkers are structurally stable and do not break under external stimuli in circulation or within target cells. Payload release typically relies on antibody degradation in lysosomes, known as a "complete degradation release" mechanism. Typical types include:
Advantages and applications:
Representative product: T-DM1 (Kadcyla) uses a non-cleavable SMCC linker to release the Lys-MCC-DM1 metabolite via antibody degradation.
Non-cleavable linkers are suitable for:
Explore our wide range of high-quality ADC linker products, including cleavable, non-cleavable, stimuli-responsive, and multifunctional options. Tailored for stability, precise payload release, and research-ready applications.
The functional groups of a linker directly determine the selectivity, stability, and drug-release behavior of ADC conjugation reactions, making them core elements for constructing high-quality ADC molecules. By carefully selecting and designing typical functional groups such as maleimides, NHS esters, hydrazones, disulfides, and carbamates, ADC structural uniformity, plasma stability, and release kinetics can be significantly optimized.
The hydrophilic or hydrophobic nature of the linker directly affects ADC physicochemical properties and in vivo behavior, including solubility, stability, aggregation tendency, and pharmacokinetics.
Hydrophilic Modifications
Hydrophobic Modifications
Overall, balancing hydrophilic and hydrophobic properties is critical for ADC stability, reduced immunogenicity, and optimized tissue distribution.
The chemical mechanism of the functional group determines its cleavage rate in physiological and pathological environments, affecting drug release timing, location, and efficiency.
Fine-tuning functional groups allows precise control over release rates, tissue specificity, and cellular uptake, a key strategy for optimizing the ADC therapeutic window.
Payload conjugation strategies are critical for ADC uniformity, stability, and efficacy. With the evolution of ADC technology, methods have progressed from early random conjugation to site-specific approaches with controlled DAR and high homogeneity. Different strategies affect not only ADC structural uniformity but also pharmacokinetics, intracellular processing, and overall therapeutic window.
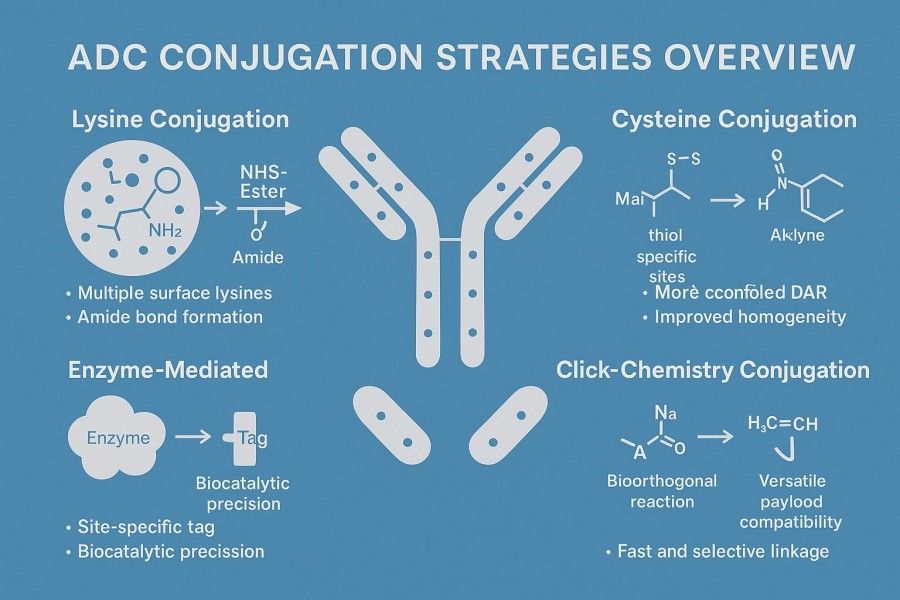 Fig. 2. ADC conjugation strategies (BOC Sciences Authorized).
Fig. 2. ADC conjugation strategies (BOC Sciences Authorized).
Lysine conjugation utilizes abundant lysine residues on the antibody surface via reactive groups such as NHS esters to form stable amide bonds. Key features:
Lysine conjugation is suitable for early screening or ADCs with low structural uniformity requirements but is gradually being replaced by more precise methods.
Cysteine conjugation exposes thiols by reducing native antibody disulfides and reacts with maleimide or pyridyl-maleimide groups to form thioether bonds.
Advantages:
Notes:
Cysteine conjugation is widely used in commercial ADCs and is the mainstream choice for highly stable, homogeneous ADCs.
Enzyme-mediated strategies (e.g., Transglutaminase, Formylglycine-generating enzyme, Sortase A) enable stable bond formation at specific antibody sites and represent third-generation site-specific conjugation technologies.
Features:
Typical methods:
Enzyme-mediated conjugation is favored in advanced ADC platforms, improving tissue distribution and efficacy consistency.
Click chemistry offers high selectivity, rapid reaction, and excellent biocompatibility, representing a key direction in future ADC development. Common bioorthogonal reactions include:
1. Azide–Alkyne Cycloaddition
2. Tetrazine–TCO Click
3. Strain-Promoted Reactions
Advantages:
In modern ADC development, the synergistic design of linkers and payloads is a core determinant of efficacy, stability, safety, and pharmacokinetic properties. An ideal linker–payload system should feature high stability, controllable release, strong tissue selectivity, manageable toxicity, and compatibility with antibody structures. Rational design not only expands the therapeutic window but also significantly enhances the commercial feasibility of ADCs.
Payloads are often highly reactive or hydrophobic, and their compatibility with the linker directly impacts conjugation efficiency, storage stability, and final product homogeneity. Key design principles include:
Spacers between the linker and payload play a critical role in modulating spatial conformation, enhancing drug solubility, and reducing aggregation risks. Optimization strategies include:
Rational spacer design significantly enhances ADC pharmacokinetic performance and is a key tool in modern ADC optimization.
Off-target toxicity is a major limitation on ADC dosing and safety. Structural optimization at the linker–payload level can markedly reduce systemic toxicity and widen the therapeutic window. Key strategies include:
Linker chemistry not only defines ADC molecular structure and functional attributes but also profoundly affects manufacturability, batch consistency, and commercial viability. From lab development to industrial-scale production, linker–payload systems face multiple process and analytical challenges that require systematic optimization and stringent quality control to ensure safety, stability, and batch uniformity.
Linker chemistry is highly sensitive to temperature, pH, solvent type, and reductant concentration.
The complex structural composition of ADCs requires precise analytical tools for quality monitoring.
Linkers and payloads are often highly hydrophobic, affecting antibody solubility and aggregation.
Linker chemistry directly impacts ADC heterogeneity, a key factor in quality consistency.
Transitioning from lab-scale to industrial-scale production must address process inconsistencies and quality fluctuations.
BOC Sciences has extensive experience in ADC linker and conjugation chemistry, integrating chemical design, payload synthesis, site-specific conjugation, and analytical characterization. With advanced technology platforms and expert teams, we provide high-homogeneity, high-stability, and highly controllable ADC solutions for clients, supporting multi-stage needs from early R&D to commercial production. Our services focus on structural optimization and controlled drug release while ensuring manufacturability and batch consistency, accelerating project timelines and enhancing ADC efficacy and safety.
References
From cytotoxins to linkers, explore our cutting-edge products for your ADC project.
| Catalog | Name | CAS | Price |
| BADC-01122 | Doxorubicin-SMCC | 400647-59-8 | Bulk Inquiry |
| BADC-01147 | DSS Crosslinker | 68528-80-3 | Bulk Inquiry |
| BADC-01135 | Fmoc-N-amido-PEG4-propionic acid | 557756-85-1 | Bulk Inquiry |
| BADC-00933 | DBCO-NHS ester | 1353016-71-3 | Bulk Inquiry |
| BADC-00453 | Mal-PEG2-NHS ester | 1433997-01-3 | Bulk Inquiry |
| BADC-00498 | Propargyl-NHS Ester | 1174157-65-3 | Bulk Inquiry |
| BADC-00364 | Fmoc-Val-Cit-PAB | 159858-22-7 | Bulk Inquiry |
| BADC-00698 | Boc-Val-Cit-PAB-PNP | 870487-10-8 | Bulk Inquiry |
| BADC-00501 | Mc-Val-Cit-PABC-PNP | 159857-81-5 | Bulk Inquiry |
| BADC-00382 | Bis-PEG1-NHS ester | 65869-64-9 | Bulk Inquiry |
Explore our advanced tools and expertise for next-generation ADC research and development.
Potent cytotoxins designed for targeted antibody-drug conjugate therapy.
Stable, selective linkers enabling precise drug release in ADCs.
Integrated payload-linker solutions for efficient and targeted ADC delivery.
Targeted therapeutics combining antibodies with cytotoxic drugs for precision treatment.










